Diagnostic tools in surgically treated patients with infective valve endocarditis
Introduction
Infective valve endocarditis (IE) is associated with high mortality and complication rates (1,2). Its long treatment and in some cases, the management of complications such as neurologic dysfunction or large septic spleen embolism followed by a splenectomy, has significant costs to the healthcare system (3).
Early diagnosis and treatment can prevent the most negative consequences (4), but prompt diagnosis remains a challenge, mainly because of the difficulties in identifying the disease due to the wide and sometimes unspecific clinical presentations. This affects the time in which a patient is referred to appropriate specialists to treat the disease, causing delays in treatment, which is often associated with advanced infection and higher mortality and morbidity (4,5).
Uncontrolled local infection, large vegetations with high risk of embolism, and heart valve failure are the main indications for surgery (6). In such cases of advanced disease, it is essential to thoroughly investigate patients with a variety of diagnostic tools; this full evaluation will directly impact decision making and management of these complicated patients and afford valuable prognostic information.
This article aims to present findings from our implementation of important diagnostic tools and discuss the diagnostic process for patients with IE who underwent surgical treatment in our institution between 1994 and 2017.
Methods
Between December 1994 and January 2017 a total of 2,458 consecutive patients with IE underwent surgery at our institution. IE was defined according to the modified Duke criteria (7) and the indication for surgery was guided by the European Society of Cardiology (ESC) guidelines for infective Endocarditis (6). For this single-center retrospective study, we analyzed clinical, microbiological, echocardiographic, and multi slice computer tomographic (MSCT) features using IBM™ SPSS Statistics Version 20.0.0.
The differences were tested using a Chi square test as appropriate for binomial/multinomial data. A P value of <0.05 was considered statistically significant.
Results
Over the study period a total of 2,458 patients with IE underwent surgical treatment in our institution. The mean age was 62 years old and 30% were women. The mean left ventricle ejection fraction (LVEF) was 55%; in one third of the cases it was less than 50% and in almost 10% of cases it was less than 30%. Patient characteristics are described in Table 1.
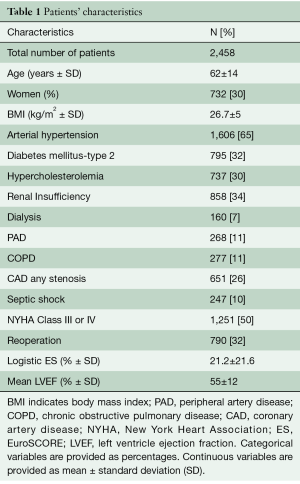
Full table
Left sided IE was more common than right sided (2,261 versus 120 patients). The most often isolated involved valve was the aortic valve (59% of all isolated valve IE). Two or more infected valves were present in 19% of the patients. Prosthetic valve IE (PVE) was present in about 28% of all cases. Aortic valve prostheses were the most commonly infected (69% of all PVE).
Concomitant cardiac surgery was done in 44% of patients—most commonly, coronary artery bypass graft surgery (CABG, 407 patients). A group of 576 patients underwent surgery of the aortic root.
The most common valve failures were mitral insufficiency (MI) and aortic insufficiency (AI) (65% and 57%, respectively). Importantly, 48% of all aortic and 35% of all mitral valve insufficiencies were severe (grade 3 or 4). There was no significant difference in the in-hospital mortality for patients with severe aortic or mitral insufficiency (5.4%, N=132 for AI and 3.4%, N=84 for MI) versus the group with no or mild insufficiency (43%, N=1,060 for AI and 35%, N=869 for MI) (P values >0.05).
The original infection focus could be identified in 51% of patients. The most common origin of IE was a dermatological focus or wound infection.
The mean length of the vegetation was 0.8 cm and 62.8% (N=1,401) of all vegetations assessed with echocardiography (N=2,230) were over 1 cm. Six patients had a vegetation larger than 3 cm. Patients with vegetations ≥1 cm had significantly more systemic embolization (P<0.001).
Septic embolization was common affect 1,081 patients (44%), the most common site of embolization was the spleen, followed by the brain. Almost one third of the patients had preoperative neurological dysfunction, which was significantly associated with brain embolization (P<0.001).
A positive blood culture was seen in 1,939 patients (79%), the most common isolated bacteria was Staphylococcus aureus. The in-hospital mortality in patients with Staphylococcus aureus was significantly higher than in the group affected by other pathogens (18.2% vs. 13.4%, P=0.004).
Discussion
Once a patient with IE is referred to our department to undergo surgery, a 12-lead electrocardiogram, complete routine laboratory tests, a Doppler of the carotid arteries, and a coronary angiography are performed as part of the standard tests ordered for every cardiac surgery patient in our institution. In IE patients, invasive diagnostic angiography is almost always done per guidelines (8). Emergency surgery cases and the few very large (>3 cm) and mobile aortic vegetations are the only exceptions. For these cases we use a MSCT to assess the coronary status as recommended (8).
Diagnostic and pre-operative workup is completed according to the endocarditis team, typically, with new blood cultures on admission, a transesophageal echocardiography (TOE) and a full-body MSCT. With this information, we decide the timing of surgery and the makeup of surgical team for each operation. For patients with a prosthetic valve or a device and no clear standard images, we do a positron emission tomography (PET)-CT to improve the accuracy of the diagnosis.
The complexity of endocarditis patients with multi-organ system impairment, requiring radiologic evaluation, and complex antibiotics therapies makes a multi-disciplinary team of experts the best approach to managing IE. In previous publications, this approach has demonstrated an improvement in outcomes (9-11). Our institution’s endocarditis team consists of a multidisciplinary group of cardiologists, surgeons, anesthetists, radiologists and intensivists who evaluate and treat each patient individually based on their disease.
In the present study, Staphylococcus aureus was the most common pathogen identified in the blood cultures and its presence could also be associated to a higher mortality. These findings are concordant with previous publications (12) that show that the aggressive course of the disease associated with this pathogen, typically with rapid development of complications and a direct impact on mortality.
Persisting positive blood cultures 48–72 hours after starting antibiotics indicate poor prognosis (13). Given this, we take new blood cultures at the admission for every single patient as a standard measure, even though most patients are referred to our institution with a known pathogen in the blood cultures and an established antibiotic therapy for at least the last 3 days.
Echocardiography plays a central role in the modified Duke diagnostic criteria (14). Transthoracic echocardiography (TTE), has a sensitivity of 70% and 50% for the diagnosis of vegetations in native and prosthetic valves, respectively. This can be improved by the use of TOE, a method with 96% and 92% sensitivity respectively (15,16). Thus, TOE has an important role in small vegetations and prosthetic valves.
As recommended for every patient with IE undergoing surgical therapy (17), an intraoperative TOE is routine in our center. Local complication assessment, especially by aggressive pathogens like Staphylococcus aureus or patients with PVE (the case for one third of patients in our series) is very important in planning surgery. For example, when a local aortic root abscess is diagnosed before the operation, a root replacement can be planned. This was partially represented in the group of 576 patients who underwent aortic root replacement.
As described in previous studies (14,18), left sided IE with severe valve dysfunction is also a predictor of poor outcome identifiable from echocardiography. Our results show that the most common valve failures were from left sided valves (65% mitral valve insufficiency and 57% aortic valve insufficiency). In this context, it is especially important to note that 48% of all aortic and 35% of all mitral insufficiencies were severe (grade 3 or 4). Despite these findings, the statistical analysis failed to show significant differences in the in-hospital mortality of the group with severe aortic or mitral insufficiency versus the group with no or mild insufficiency (P>0.05). This may be because of the small number of patients in the subgroups.
Determination of LVEF using echocardiography is important for prognosis (15,16). The mean LVEF was 55% and in one third of all patients it was <50%. Nine percent (N=221) of patients had a LVEF <30%. This was associated with higher in-hospital mortality (P<0.001).
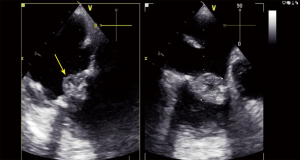
As described above, an important factor in the risk of embolization and in the indication for surgery is the length of the vegetations (13,19). In this study, the mean length of vegetations in our sample was 0.8 cm. 63% of all vegetations examined by echocardiography were over 1 cm and only 6 patients had a vegetation larger than 3 cm. Patients with vegetations of ≥1 cm had a significantly greater rate of embolization (P<0.001)—this result was in keeping with other published data (17) (Figure 1).
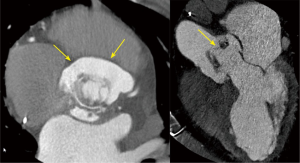
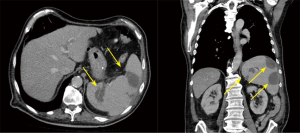
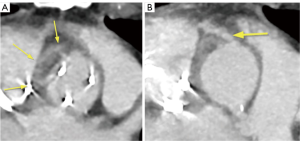
The preoperative complete body MSCT is also a fundamental part of our standard diagnostic methods. It is useful in describing the anatomy and detecting local complications like abscesses, pseudo aneurysms and fistulae (20), which are very important factors to consider when deciding on the best surgical strategy (Figure 2,3). Abscesses and infarcts of target organs, such as the spleen and the brain, can also be assessed in the MSCT images. In the case of a relevant septic embolism in the spleen, a splenectomy is performed subsequently by our abdominal surgeons during a separate surgery. This reinforces the important concept of multidisciplinary team management for these patients (Figure 4).
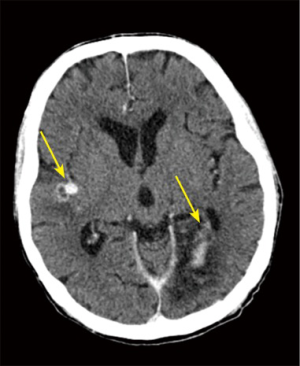
We determined that the septic embolism rate in our patients was 44% (N=1,081) of the whole group and the most common sites were the spleen and the brain, which was also consistent with previous studies (21). If the patient presents a septic embolism in the brain, the MSCT helps detect any important hemorrhagic transformation, which would delay the time of the surgery as recommended (22,23). In this study, almost one third of the patients had preoperative neurological dysfunction, which was significantly associated with brain embolization (P<0.001) (Figure 5).
The long study period with a sample of 2,458 patients could be seen as an advantage, however, recent changes such as the creation of an endocarditis team, improvement of diagnostic tests, and surgical experience, the presented results could have significant variations among the patients who had their surgery at the beginning and at the end of the analyzed period, which is an indication for future studies in our institution.
This study is also retrospective which means there is a risk of confounding and bias is more likely to be present when compared to prospective studies.
As a single center experience, it is difficult to reproduce the results of our study given procedural and management differences which are often unique to each centre. Multi-centre, prospective studies are indicated to validate results with regards to management of IE.
Conclusions
Echocardiography is the most important diagnostic tool used for our patients with IE given the valuable diagnostic and prognostic information it provides. Preoperative CT contributes to the entire decision-making process, before, during and after surgery. Blood cultures are mandatory in choosing and adjusting the antibiotic therapy. Coronary angiography can be safely done in most cases despite vegetations on the aortic valve. We use PET CT only in suspected prosthesis or device-related IE and only in addition to our standard diagnostic tools when these are not congruent with the clinical picture. A multidisciplinary evaluation and management by an endocarditis team is mandatory for these complex patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leone S, Ravasio V, Durante-Mangoni E, et al. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection 2012;40:527-35. [Crossref] [PubMed]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. [Crossref] [PubMed]
- Sunder S, Grammatico-Guillon L, Baron S, et al. Clinical and economic outcomes of infective endocarditis. Infect Dis (Lond) 2015;47:80-7. [Crossref] [PubMed]
- Aksoy O, Sexton DJ, Wang A, et al. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis 2007;44:364-72. [Crossref] [PubMed]
- Middlemost S, Wisenbaugh T, Meyerowitz C, et al. A case for early surgery in native left-sided endocarditis complicated by heart failure: results in 203 patients. J Am Coll Cardiol 1991;18:663-7. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013;112:1171-6. [Crossref] [PubMed]
- Lancellotti P, Rosenhek R, Pibarot P, et al. ESC Working Group on Valvular Heart Disease position paper—heart valve clinics: organization, structure, and experiences. Eur Heart J 2013;34:1597-606. [Crossref] [PubMed]
- Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009;169:1290-8. [Crossref] [PubMed]
- Chirouze C, Cabell CH, Fowler VG Jr, et al. Prognostic factors in 61 cases of Staphylococcus aureus prosthetic valve infective endocarditis from the International Collaboration on Endocarditis merged database. Clin Infect Dis 2004;38:1323-7. [Crossref] [PubMed]
- Lopez J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013;34:1749-54. [Crossref] [PubMed]
- Chu VH, Cabell CH, Benjamin DK Jr, et al. Early predictors of in-hospital death in infective endocarditis. Circulation 2004;109:1745-9. [Crossref] [PubMed]
- Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010;11:202-19. [Crossref] [PubMed]
- Mugge A, Daniel WG, Frank G, et al. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol 1989;14:631-8. [Crossref] [PubMed]
- Shapira Y, Weisenberg DE, Vaturi M, et al. The impact of intraoperative transesophageal echocardiography in infective endocarditis. Isr Med Assoc J 2007;9:299-302. [PubMed]
- San Roman JA, Lopez J, Vilacosta I, et al. Prognostic stratification of patients with left-sided endocarditis determined at admission. Am J Med 2007;120:369-7. [Crossref] [PubMed]
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366:2466-73. [Crossref] [PubMed]
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53:436-44. [Crossref] [PubMed]
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005;112:69-75. [Crossref] [PubMed]
- Yoshioka D, Sakaguchi T, Yamauchi T, et al. Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann Thorac Surg 2012;94:489-95. [Crossref] [PubMed]
- Eishi K, Kawazoe K, Kuriyama Y, et al. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg 1995;110:1745-55. [Crossref] [PubMed]