Surgical techniques of total arch replacement using selective antegrade cerebral perfusion
Introduction
This detailed illustrated article describes our preferred surgical technique of total arch replacement using selective antegrade cerebral perfusion (SACP). Our current approach includes: (I) meticulous selection of arterial cannulation site and type of arterial cannula; (II) SACP for neuro-protection; (III) whole body hypothermia with minimal tympanic temperatures between 20 and 23 °C and minimal rectal temperatures below 30 °C; (IV) early re-warming after distal anastomosis with SACP flow adjustment while monitoring brain oxygenation by near infrared spectroscopy (NIRS); and (V) after 2006, maintaining strict fluid balance below 1 L by the extracorporeal ultrafiltration method (ECUM) during cardiopulmonary bypass (CPB), with the expectation of more rapid pulmonary functional recovery.
Operative techniques
Preparation
The patient is placed in the supine position and the diodes of NIRS are attached on the foreheads bilaterally. For NIRS, we use INVOS 5100C (Somanetics, Troy, MI), which provides continuous regional cerebral oxygen saturation (rSO2). The rSO2 readings are expressed as an index, measuring differences from an unknown baseline. A standard median sternotomy is performed and there is no need for extension of the skin incision to the left neck.
Before going on the CPB, the innominate vein is fully mobilized with division of several branches to facilitate exposure of the aneurysm. The vein is seldom divided (Figure 1). Dissection of the aneurysm, aortic arch branches, or the vagus nerve is not usually performed to minimize aortic manipulation and recurrent laryngeal neuroprexia. Taping around the arch or arch vessels is not necessary (Figure 2). The serrated balloon tipped cannulae usually sit well in the neck vessels without snaring or can be fixed with a silk suture.
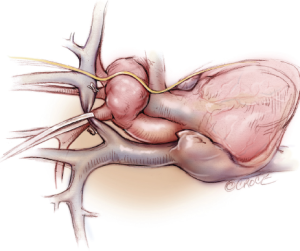
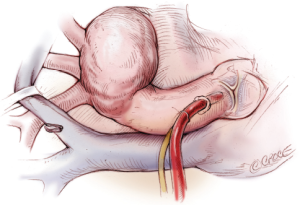
Cannulation
Preoperative CT scan is done in every patient to assess the atheromatous lesions in the ascending aorta. Both transesophageal and epi-aortic echocardiography are applied to interrogate the ascending aorta and determine cannulation site. CPB is established with bi-caval drainage. The left ventricle is vented through the right upper pulmonary vein. Femoral artery cannulation is applied particularly in patients with aortic dissection. For the diseased ascending aorta/aortic arch, a 24 Fr dispersion arterial cannula (Duraflo II, Edwards Lifesciences LLC, Irvine, CA) is used. Otherwise a straight tip cannula in the ascending aorta (DLP, Medtronic, Minneapolis, MN, USA) is used. Cannula tip is always set towards the aortic valve to avoid direct flow to the arch (Figure 2). All patients receive 100 mg of betamethasone sodium phosphate, and 100 mg of sivelestat sodium hydrate is added to the pump circuit at the initiation of CPB.
Exposition
After tympanic temperature has dropped down to 23 °C with rectal temperature below 30 °C, the aortic arch aneurysm is opened while raising the central venous pressure to 10 mmHg or brief periods of retrograde cerebral perfusion. By using four traction sutures, including one at the root of each arch vessel and one in the lesser curvature of the arch, the inside of the aortic arch is exposed (Figure 3).
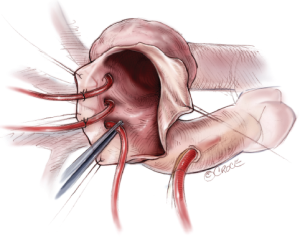
SACP is then initiated. A 14- or 16-Fr balloon-tipped cannula is inserted from inside the aorta into the brachiocephalic artery, and 12-Fr cannulae are positioned in the left common carotid and left subclavian arteries individually. We routinely use self-inflating serrated-balloon-tipped cannulae (Fuji systems, Tokyo). Snaring of neck vessels is strictly prohibited. Stay stitches are applied to secure the cannulae (Figure 3). After insertion, cannulae are fixed to the right skin edge towels, to prevent hindering exposure of the orifice of the descending aorta. Avoiding cerebral embolism is particularly important in SACP cannulation. For the ostium of severely atherosclerotic arch vessels, arteriotomy is extended distally from the diseased ostium so that cannulae can be placed in position under direct vision. SACP flow is maintained at 10-12 mL/kg/min using an independent roller pump, and balloon tip pressure is maintained between 30 and 40 mmHg. Myocardial protection is achieved by antegrade or retrograde cardioplegia. Gauze sponges are placed in the aortic root and in the descending aorta to catch atheromatous debris.
Aortic arch reconstruction
The vagal nerve is never dissected out to prevent left recurrent laryngeal nerve injury and to alleviate inadvertent injury of the esophagus. Direct circumferential transection of the proximal end of the descending aorta, distal to the aneurysm is usually performed starting at 3 o’clock position. Incising back of the aortic arch may not be necessary and can be rather harmful sometimes to the left recurrent laryngeal nerve (Figure 4). It is very important not to lose the adventitia in this maneuver. Further dissection of the descending aorta, at least 3-5 cm, is necessary in order to have enough suture bites and for placement of a 25 mm wide Teflon felt strip around the proximal descending aorta. Usually the bronchial arteries are divided, and sometimes the upper intercostal arteries, in order to mobilize the descending aorta (Figure 5). The thoracic duct often lies along the descending aorta in this level and should not be injured. Every attempt is made to leave the left pleura intact otherwise inadvertent bleeding from the distal anastomosis may escape into the deep in the left pleural cavity and go unnoticed.
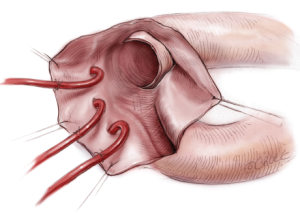
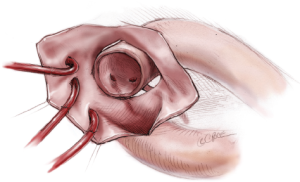
A sealed four-branched (10, 8, 8, 8 mm) graft (J graft. Japan Lifeline, Tokyo or Triplex, Terumo Corporation, Tokyo, Japan) is used. Open distal aortic anastomosis is performed with a flexible sucker being placed inside the graft to collect blood in the descending aorta (Figure 6). A 4-0 monofilament suture on a 22 mm needle is used. The suture starts at 9 o’clock position, progressing counter-clockwise. Suture is tied first and bites are taken at least 10 mm apart. After completing the anastomosis, a 4-0 compacting suture is placed to fasten the Teflon felt (Figure 6).
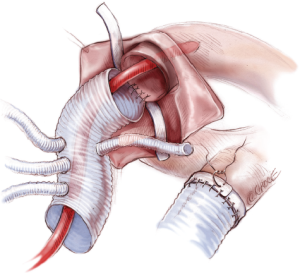
Lower body circulation is then reinstituted through one branch of the graft, sometimes after flushing from the femoral arterial cannula, and whole body rewarming is started with antegrade perfusion (Figure 7). At this stage, secure hemostasis in the distal suture line must be obtained. Concurrent with re-warming, SACP flow is gradually increased while maintaining the baseline values of rSO2. However, SACP flow is limited below 1,200 mL/min at all times to prevent brain edema.
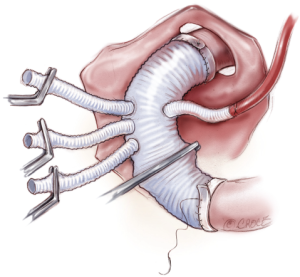
Proximal anastomosis is then accomplished using 4-0, 17 or 22 mm needle, monofilament suture with 10 mm wide Teflon felt reinforcement. After the heart is de-aired, the graft clamp is released and coronary reperfusion is resumed.
Epi-aortic vessel reconstruction
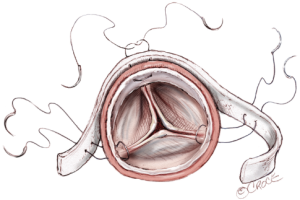
Aortic arch is divided to make vessel buttons with each traction suture (Figure 8). Three arch vessels are reconstructed tandem to the graft branches using a 5-0, 17 mm needle, monofilament suture. Usually the parachute technique is used and each vessel is perfused after completion of the anastomosis. CPB is then weaned-off. The left pleura is now opened longitudinally near the sternum to monitor unexpected bleeding into the left pleural cavity.
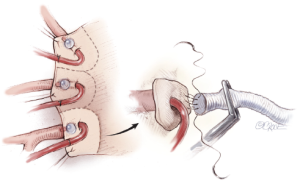
If the patient also has carotid artery or intracranial artery stenosis/occlusion, epi-aortic vessel reconstruction is performed prior to re-warming (Figure 9). After distal anastomosis to the descending aorta, while perfusing the brain at 23 °C, the left subclavian, left common carotid, and brachiocephalic arteries are anastomosed and perfused. Rewarming is then started and proximal anastomosis is performed in the same fashion.
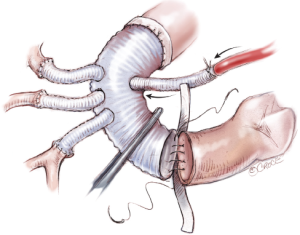
Aortic dissection
In patients with acute aortic dissection, requiring total arch replacement, hypothermic circulatory arrest is achieved and the arch is opened. SACP is initiated by inserting three balloon-tipped catheters in the true lumen of the arch branches. No tourniquet around the arch vessels is necessary. The arch is transected just distal to the left subclavian artery and the adventitia of the descending aorta is carefully dissected without injuring it (Figure 10).
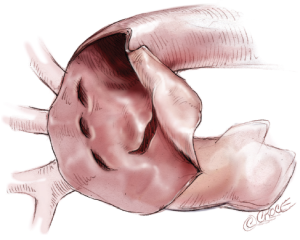
A 5 cm long, 16 to 20 mm wide, Dacron graft is inserted in the true lumen of the descending aorta and a wider, 3 cm, Teflon felt strip seated outside the adventitia (Figure 11). A continuous 5-0 polypropylene horizontal mattress suture with straight needle is used to secure the elephant trunk, dissection flap, and the adventitia (Figure 12). At this stage, all circulation is stopped for 3 minutes to dry-up the lumen of the descending aorta, and biological glue (Bioglue) is used to obliterate the false lumen.
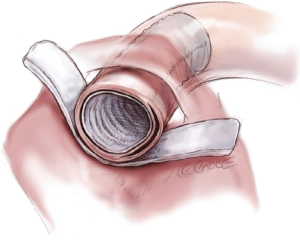
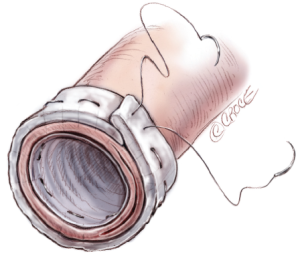
Distal anastomosis with four-branched graft to the descending aorta, including the elephant trunk, aortic wall, and the felt strip, is then performed using 4-0 sutures (Figure 13).
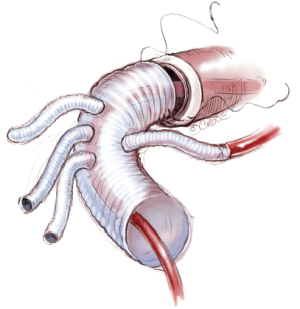
The circulation of the lower body is then initiated. The proximal aortic stump is incorporated with the aortic valve commissures, the false lumen, and the inner and outer Teflon felt using same 5-0 polypropylene horizontal mattress sutures (Figure 14). Proximal anastomosis is done in the same manner. Aortic arch branches are also reconstructed using small pieces of Teflon felt strips.
The fluid balance during CPB is strictly controlled and kept below 1,000 mL by the ECUM (Capiox Hemoconcentrator HC11, Terumo Co., Tokyo, Japan). After completion of total arch replacement, the patient is weaned off CPB, receives protamine and decannulated as per routine.
Comments
For surgical approaches to the arch aneurysm, especially for the distal arch aneurysm, both median sternotomy and left thoracotomy can be applied. However, we have been using median sternotomy exclusively to avoid several complications associated with left thoracotomy. Patients with chronic obstructive lung disease or chronic aortic dissection tend to have some adhesions between the aneurysm and the left lung. Lung injury secondary to surgical manipulation, especially after deep hypothermia, can add further risk to the postoperative pulmonary complications (1). Also, the incidence of left recurrent laryngeal nerve damage was higher in patients who had left thoracotomy than those with midsternotomy. We reported that the level of carina or 17 cm from the sternum level to the back was approachable from the median sternotomy (2).
The selection of an arterial cannulation site and type of cannula are very important in preventing atheroembolic events, particularly neurological complications. In most instances we select the ascending aorta as an arterial cannulation site after inspection of the epiaortic echographic scan. In an experimental study, Fukuda et al. (3) confirmed that directing the cannula tip of the Dispersion cannula towards the aortic root generated slower and less turbulent flow in the transverse arch of the glass models of both healthy and aneurysmal aortic arches.
Selective antegrade cerebral perfusion is now considered to be most reliable brain protection method and has been widely used in the field of aortic surgery, albeit with variations (4,5). We have always used three cannulae, which are inserted from inside of the arch without snaring. Urbansky et al. (6) used only one cannula to perfuse the whole brain and reported a low incidence of postoperative stroke. Many surgeons perfuse only the brachiocephalic and left common carotid artery, and not the left subclavian artery. However, incompleteness of the circle of Willis has been reported as 20% to 30% in normal population(7), with the vertebral arteries sometimes hypoplastic or stenotic, especially in elderly patients. Also the left subclavian artery often supplies collateral vessels to the spinal cord.
As noted by Kouchoukos et al. (8), the exclusion technique for aortic anastomosis or aortic branches is securer than the inclusion technique. We always transect the aorta just distal from the aneurysm and divide several bronchial arteries. This may also alleviate inadvertent injury to the esophagus or left lung. Technically, transection of the aorta is usually performed by incision circumferentially from inside of the aorta, not by incising the aortic arch wall anteriorly or posteriorly.
Usage of the four-branch graft has several potential advantages over the “island” aortic cuff technique to reconstruct the arch vessels. Individual anastomosis of each arch vessel can provide secure anastomosis. By also dividing the arch cuff into the three buttons, more liberal exposure of the aortic arch can be obtained. Consequently, proximal anastomosis of the ascending aorta can precede the arch anastomosis and the resultant reduction in the cardiac ischemic time is beneficial.
In summary, we have found that SCAP is a reliable neuro-protection strategy during aortic arch surgery. In our hands, SACP is associated with low perioperative morbidity and mortality.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Asimakopoulos G, Smith PL, Ratnatunga CP, et al. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 1999;68:1107-15.
- Asano M, Okada K, Nakagiri K, et al. Total arch replacement for aneurysm of the aortic arch: factors influencing the distal anastomosis. Interact Cardiovasc Thorac Surg 2007;6:283-7.
- Fukuda I, Fujimori S, Daitoku K, et al. Flow velocity and turbulence in the transverse aorta of a proximally directed aortic cannula: hydrodynamic study in a transparent model. Ann Thorac Surg 2009;87:1866-71.
- Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg 1999;67:1874-8; discussion 1891-4.
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9.
- Urbanski PP, Lenos A, Lindemann Y, et al. Carotid artery cannulation in aortic surgery. J Thorac Cardiovasc Surg 2006;132:1398-403.
- Merkkola P, Tulla H, Ronkainen A, et al. Incomplete circle of Willis and right axillary artery perfusion. Ann Thorac Surg 2006;82:74-9.
- Kouchoukos NT, Wareing TH, Murphy SF, et al. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann Surg 1991;214:308-18; discussion 318-20.





