Functional and pathomorphological anatomy of the aortic valve and root for aortic valve sparing surgery in tricuspid and bicuspid aortic valves
Introduction
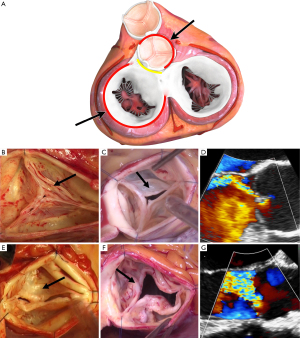
The functional aortic valve (AV) apparatus is a geometric structure with close relationships between the AV and the aortic root (1). The aortic root thus constitutes the native stent of the AV and can also be described as the three-dimensional functional aortic annulus (FAA); composed of the aorto-ventricular junction (AVJ), the sino-tubular junction (STJ), and both junctions are inter-connected through the insertion line of the AV cusps. Loss of integrity of any of these components leads to AV dysfunction, regurgitation or even stenosis. Moreover, based on the principles of the mechanism of mitral regurgitation set forth by Alain Carpentier, Gebrine El Khoury has developed the functional classification for aortic regurgitation (AR) during the late 90’s and early into the new millennium (Figure 1) (2,3).

The El Khoury classification thus explains all the different types of AR and illustrates the loss of the geometric relationship between AV-structures. Hence, in analogy to Carpentier’s classification: AR type I corresponds to dilation of the FAA; type II corresponds to an excess of leaflet motion (prolapse); and type III to restricted leaflet motion. In type I, dilatation can be limited to the distal portion of the FAA, the STJ (type Ia), extend to the entire FAA (type Ib), or be limited to the proximal potion of the FAA, the AVJ (type IC). In type I, AR is secondary to the disproportionate FAA dilation relative to leaflet size, with a loss of central coaptation, because the leaflets are pulled outward. It is important to note that a compensatory mechanism exists at the level of the leaflets so that in most patients, and to a certain extent of FAA dilatation, the AV-leaflets can stretch and thus increase in size to accommodate for the FAA dilatation and remain functional and competent (4,5). In FAA dilatation, especially type Ia and Ib, the leaflets are stretched up- and outward, so that the coaptation height is abnormally high and their motion is restricted, as in mitral leaflets in dilated or ischemic cardiomyopathy (Carpentier classification type IIIb). This functional restriction (with “normal” AV leaflet tissues) needs to be differentiated from the organic restriction caused by leaflet thickening and retraction of the type III AR.
In type II, AR is secondary to the disproportionate elongation of the leaflet free margins relative to the FAA diameter, and in comparison, to the other two leaflet’ free margins (4). The consequence is that the elongated leaflet free edge drops below the level of the others, with a loss of coaptation (a prolapse) and resultant eccentric AR. A prolapse can affect more than one leaflet. Finally, in type III, AR occurs due to restricted leaflet motion and fibrous thickening, calcification or leaflet retraction. These lesions, if severe or diffuse, can certainly also induce AV stenosis. The different mechanisms of AR can be isolated, but they more frequently occur simultaneously in patients with AR especially in cases of long-standing severe AR, large aneurysmal disease or congenital AV disease. Of note, type Ic AR is almost always associated with type II, because the leaflets are pulled outward from their nadir, which decreases the coaptation height, increases stress on the leaflets, and favors the appearance of a leaflet prolapse (6).
The goal of AV-sparing surgery and repair is to restore the geometric relationship between the different components of the AV and root, and also to stabilize the repair, which improves long-term durability. Therefore, the surgical toolbox should entail techniques to repair or remodel any component of the AV/root complex; valve-sparing root replacement techniques hereby represent a major tool in the available surgical armamentarium. Herein, we review the normal and pathomorphological anatomy of the AV and root, with particular regard to AV-sparing surgeries and AV-repair. We compare direct valve measurements to modern imaging technology, to analyze these structures and their pathologies. The aim is to provide surgeons with a comprehensive review of the AV and root anatomy, and how these structures need to be approached and remodeled during valve-sparing procedures.
The aortic root
Normal aortic root dimensions
In adults, the normal aortic root size is a useful reference. Thus, the goal of AV-repair or valve sparing-surgery is frequently to restore normal dimensions in dilated aortic root segments with annuloplasty or an aortic graft. The root dimensions are generally measured at three different levels, the AVJ (proximal), the middle of the sinus of Valsalva (middle) and the STJ (distal). In an adult population, the average diameter of the AVJ is 26 mm in males, 23 mm in females (upper normal limits 30 in males, 26 in females), the average diameter of the sinus of Valsalva is 34 mm in males, 30 mm in females (upper normal limits 40 in males, 36 in females), and the average diameter at the STJ level is 29 mm in males, 26 mm in females (upper normal limits 36 in male, 32 in females). These measurements are taken with two-dimensional-echocardiography in long axis view at end-diastole. The AVJ and sinus of Valsalva dimension correlates most closely with body surface area, and the STJ (like the ascending aorta diameter) correlate most closely with age (7,8). Moreover, a more recent study showed a strong independent association between aortic root size with age, body size and gender (9). On this note, aortic root diameter was larger in men and increased with body size and age.
AVJ dilatation
The AVJ, under normal circumstances, is oval-shaped with a short and a long diameter. In normal tricuspid aortic valves (TAVs), the average short diameter is 22 mm and the long diameter is 27 mm. Two-dimensional long axis view of the aortic root generally shows the shorter AVJ diameter. Three-dimensional echocardiography or computed tomography (CT) scan are used to determine short and long diameter with better precision. The short diameter is delineated from mitro-aortic curtain on one side to the interventricular septum on the opposite side, while the long diameter extends from posterior trigone to anterior. The short/long diameter ratio is near 0.8 and increases to 0.9–1 in cases of AVJ dilatation. Chronic AR and root dilatation are frequently associated. Some authors have shown that root dilatation and degree of AR increased in parallel in TAV and also bicuspid aortic valve (BAV) (10,11). The AVJ size also increases with chronic AR. In an internal cohort of patient operated on for valve repair or sparing surgery, the average AVJ diameter by two-dimensional echo varied from 23 mm in TAV patients with AR grade 0–1+ to 27 mm in TAV patients with AR grade 2+ or more. In patients with BAV, the average AVJ diameter varied from 27 mm in those with AR grade 0–1+ to 31 mm in those with AR grade 2+ or more (at our center, unpublished data).
Several studies have shown that a dilated AVJ was an important predictor for repair failure through early recurrence of AR (6,12,13). AV annuloplasty aims at reducing AVJ diameter and increases valve coaptation. Circumferential annuloplasty techniques comprise valve-sparing reimplantation, external ring, or suture annuloplasty. These have shown to improve long-term durability of AV repair by stabilizing the repair and overall improving valve coaptation over time (14-16). The pathophysiology leading to AVJ dilatation is thought to be a mix of connective tissue deficiency as part of the aortopathy, and the volume overload induced by AR.
Which segment of the AVJ circumference dilate the most is not clear yet, but we hypothesize that as in the mitral and tricuspid valve, the muscular portion of the aortic annulus dilates more than the inter-trigonal fibrous portion (part of the fibrous skeleton of the heart). This mechanism, in analogy to the mitral valve, may explain the fact that a prolapse is more frequently observed on the leaflet supported by the “dilated” muscular portion (i.e., right coronary leaflet or fused left/right leaflet in BAV) (Figure 2) (17,18). Therefore, we believe that the most important role of AVJ annuloplasty is the remodeling and stabilization of the muscular portion of the AVJ, identical to the mitral valve, where we commonly perform a trigone-to-trigone posterior mitral annuloplasty.
Topographic relationship between virtual basal ring (VBR) and AVJ
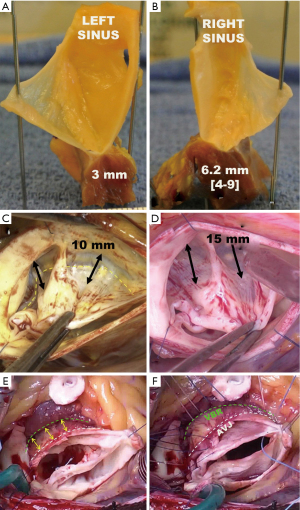
The annuloplasty aims at remodeling and stabilizing the basal portion of the AV at the nadir of the leaflet insertion. The plane passing through the AV leaflet nadirs is called the VBR. This reference plane is deeper and more proximal than certain parts of the AVJ, which represents the transition zone of the cardiac [left ventricular outflow tract (LVOT)] to aortic wall tissues. Once the aortic root is dissected, as in valve-sparing reimplantation surgery, the AVJ corresponds to the limit of the dissection and is macroscopically well-delineated on the external aspect of the aortic root. The AVJ is at the same level as the VBR along the fibrous portion of the circumference, corresponding to the mitro-aortic curtain. But along the muscular portion and the membranous septum, the AVJ stands up to half a centimeter above the VBR (Figure 3). The shape of the AVJ at the anterior part of the annulus is caused by the insertion of the ventricular myocardium into the basal portion of the left and the right coronary sinus. Thus, the muscular insertion at the base of these two sinuses form the so-called myocardial crescent, where the sinus tissues are thinner compared to the rest of the aortic root (19,20). The ventricular myocardium also extends to the lower third, or even half, of the interleaflet triangle between the left and right sinus. In the area of the membranous septum, insertion of the right atrium into the aortic wall delineates the AVJ at approximately the lower third of the right/non interleaflet triangle (21). Moreover, the thickness of the tissues at the AVJ varies along the root circumference. The tissue thickness is relatively thin, and on average, only a couple of millimeters thick along the fibrous portion and the membranous septum. However, along the interventricular septum it’s thicker and reaches a maximum at the level of the right sinus (Figure 4A,4B). At this level, tissue thickness corresponds to the width of the myocardial crescent that is on average 6 mm (20,21). In some patients with BAV, the myocardial crescent can reach up 10 or 15 mm, and in rare circumstances even more (Figure 4C,4D) (17).

Effects of VSRR
Since the annuloplasty (ring or graft) needs to be placed at the level of the VBR, and the entire circumference of the aortic root, the external root dissection has to extend below the level of the AVJ in the area of the interventricular septum. This maneuver is called the “El Khoury deep root dissection” (Figure 4E,4F) (22-24). Starting from behind the left/right commissure, the maneuver consists of separating the LV myocardium from the pulmonary trunk. A similar dissection is performed to harvest the autograft during a Ross procedure. The dissection must go 10 to 15 mm below the AVJ to reach the VBR level on the external aspect of the interventricular septum. The dissection can then be extended towards the right sinus, developing the plane between myocardial fibers of the right ventricular outflow tract (RVOT) and those of the left ventricle. From approximately the middle portion of the right sinus to the right/non-commissure, the myocardial fibers of the RVOT insert directly onto the sinus tissues. These fibers can be carefully detached from the sinus wall with the electrocautery or with scissors to extend the dissection towards the VBR level. The dissection must be performed close to the sinus wall to avoid unintentional injury to the RVOT. At the right/non commissure, the dissection is limited by insertion of the membranous septum and the proximal sutures are placed a little higher in order to avoid injury to the conduction bundle, and therefore stays above the level of the VBR (22). The tissue thickness at the level of the VBR is on average 3.3 mm, considering the thin fibrous-, and thicker muscular portion. The external annuloplasty, at the level of the VBR, will incorporate these tissues and the residual internal diameter can then be estimated using the formula: device diameter − (3.3 × 2) (21).
Coronary variations and implication for VSRR
Coronary ostia, especially in congenital AVs [BAV, unicuspid aortic valve (UAV)], can have atypical locations in the normal left or right sinus; or can have an abnormal origin from the wrong sinus or wrong coronary. Moreover, aortic root dilatation can “displace” the left coronary ostium to the left or right side, and the right coronary ostium cranially (25-27). An abnormal right coronary can arise from the left coronary sinus. These coronaries with an intramural course can be unroofed by carefully incising around the tip of the left/right commissure and carefully detaching the commissure, which then has to be re-attached to the aortic wall later. This, however, creates a neo-ostium of the right coronary artery in the right coronary sinus.
An abnormal origin of the circumflex coronary artery from the right coronary artery can also occur, which then passes around the aortic root close to the non-coronary leaflet insertion and its’ adjacent commissures. It is thus important to recognize this variant preoperatively, in order to avoid passing sutures for the annuloplasty or proximal suture line during valve-sparing procedures through the aberrant vessel. The artery can be easily detached from the base of the aortic root with subsequent safe suture placement. An abnormal left or right ostium close to a commissure requires reimplantation with a keyhole technique. The graft is trimmed in a keyhole fashion in order to attach the ostia to the commissure (28).
Aortic valve anatomy
It is important to be familiar with normal AV anatomy well enough, to then appreciate different phenotypes and recognize pathologic conditions. The AV leaflet shape and size can be easily characterized by these three measurements: leaflet height [or geometric height (gH)]; free edge length; and commissural height. The gH is a simple measurement taken at the largest portion of the leaflet from the nadir to the middle of the leaflet free edge. gH is generally used as a surrogate of leaflet size/surface and can be used as a criterion to decide on valve preservation or replacement. It can also serve as a reference to size the graft, during valve-sparing procedures. Adequate gH is necessary to consider a durable AV-sparing or AV-repair approach (29).
The gH must never be reduced during AV repair. It can be increased however, for instance, through patch extension or central plication in asymmetric BAV with partial conjoined leaflet fusion. The free edge length is measured between two commissures, along the free edge of the leaflet. A precise measurement is more difficult to acquire, considering the three-dimensional, thin and mobile aspect of the free edge. This measurement is a good surrogate however, for leaflet mobility and coaptation height. The free edge length cannot be increased but it can easily be shortened by plication in case of a prolapse, in order to increase leaflet coaptation height. The commissure height, or root height, is not a measurement of the leaflets themselves, but is representative of the valve configuration that needs to be respected during valve-sparing procedures. Commissure height is measured from the base of the interleaflet triangle to the tip of the commissure near the STJ. Commissure height must never be shortened at the risk of inducing a prolapse. It can be slightly increased by pulling on the commissure during valve sparing reimplantation. The commissural height can serve as a reference for graft sizing, for valve-sparing procedures utilizing Valsalva-type grafts.
The gH
In the normal TAV, the average gH ranges from 18 to 20 mm in three ex-vivo studies (1,18,30). In a clinical study of patients operated on for AR or aortic root aneurysm, the average gH measured intraoperatively at 20 mm for TAV and 24 mm for the non-conjoined leaflet of BAV (29). The gH correlates with body size and root size. There are small variations among the gH of the three leaflets in normal TAV. The non-coronary leaflet tends to be slightly larger followed by the left- and right coronary leaflets.
The gH can also be measured by modern three-dimensional imaging modalities, such as echocardiography or CT (31-33). Imaging allows the clinican to localize the appropriate middle portion of the leaflet at which to measure gH. In these studies however, the average gH is about 15 to 16 mm, 3 to 5 mm less compared to ex-vivo or intraoperative measurements. This difference ensues due to the fact that intraoperatively the measurement is taken while the leaflet is stretched, and during imaging, the gH is generally measured simply in diastole without a physical stretch. Unfortunately, there are no correlative studies comparing intraoperative and imaging measurements. Hence, the intraoperative assessment of the gH remains the gold-standard to evaluate the quantity of leaflet tissues. It is generally accepted that a gH of less than 15–16 mm in adults constitutes a contraindication to AV-sparing surgery or AV-repair, in TAV or BAV. In patients with small gH (e.g., 16 mm), the use of smaller Dacron grafts (size 26) should be advised for valve-sparing reimplantation.
The free edge length
In the normal TAV, the average free edge length ranges from 30 to 34 mm in four ex-vivo studies (1,18,34,35). In these studies, an average length close to 30 mm was found in specimens fixed in formalin, or when the free edge was measured after cutting the leaflet from the aortic root. An average of 34 mm was found in fresh specimens when the free edge was left attached to the aortic root and while applying tension for measurement. The free edge length correlates with body size, as well as root size. There are small differences between free edge length of the three leaflets in a normal TAV; the right coronary leaflet is slightly longer followed by the non- and left coronary leaflets. The free edge can also be measured by CT using three-dimensional reconstruction (32,33). In these studies, the average free edge length was 33 or 34 mm, very similar results compared to ex-vivo analysis where leaflets were measured on fresh specimen and left attached to the aortic root (1,18). The good correlation between direct and indirect measurements make imaging by CT a good tool to assess this important characteristic, preoperatively. Currently, graft-sizing based on free-edge length is not routinely employed, but is under investigation in a trial. The advantage of free edge length assessment: it allows for quantification and consideration of free-edge elongation, as observed in aortic root aneurysms. Leaflet parameters that are at the center of many technical errors, such as choosing a too small of a graft in patients with significant free margin elongation, can inadvertently lead to decreased leaflet coaptation and early repair failure.
gH and free edge length in root dilatation and AR
AV leaflets are pliable and can be stretched to adapt to aortic root dilatation. This mechanism has been previously demonstrated by echocardiography (5). It was noted that free edge length, gH and sinus height increase in patients with root dilatation. They also elaborated that two-dimensional echocardiography was not the most accurate means to precisely measure these thin three-dimensional structures. They reported that the average free edge length increases approximately from 31 to 38 mm; the gH increase approximately from 18 to 21 mm, and sinus height increased from 22 to 30 mm.
In 2019, we repeated a similar study in 132 patients with TAV or BAV operated on for aortic root aneurysm or AR (4). Free edge length and gH was systematically measured intraoperatively using a suture aligned to the free edge and a straight ruler. We found that free edge length and gH correlate with root size in TAV and in BAV. We also demonstrated that the free edge length increases in a much larger proportion relatively to the gH. Considering an average gH of 19 mm and free edge length of 34 mm in normal TAV, the group of patients with root dilatation had an average gH of 21 mm and an average free edge length of 44 mm. In the group of patients with prolapse, the leaflet with prolapse had an average free edge length of 48 mm compared to 42 mm for leaflets without prolapse. In BAV, free edge length was 45 mm on average in the group of patients with root dilatation and patients with a prolapse of the fused leaflet average was 57 mm, and for the non-fused leaflet 47 mm. The gH in BAV patients was generally larger in the non-fused leaflet with an average of 24 mm compared to an average of 20 mm for the fused leaflet.
These findings have several direct implications for AV-sparing-, and AV-repair surgery:
- In any root dilatation, even if the valve looks and functions normal, the leaflets are stretched and present with elongated free edges. Reduction of root size with the prosthetic graft risks inducing a prolapse (Figure 5). Therefore, avoiding graft under-sizing, resuspending the commissure as high as possible, and checking the coaptation level at the end of the procedure are the best tricks to avoid a prolapse after valve-sparing surgeries.
- A leaflet prolapse has an elongated free edge compared to adjacent leaflets without prolapse. Therefore, the free edge of the of the leaflet with prolapse can be reduced by the difference it has with regards to the leaflets without a prolapse.
- In patients with ascending aorta and STJ dilatation, remodeling of the STJ can be facilitated by measuring the leaflet free edge and dividing it by 1.3 (free edge/1.3). The resultant ratio determines the graft- or ring-size required for STJ remodeling.
- The leaflet free edge length is an informative parameter, which provides additional valuable information for clinical decision-making. It should therefore be measured routinely, similar to the gH, or effective height (eH) during AV-sparing or AV-repair surgery.
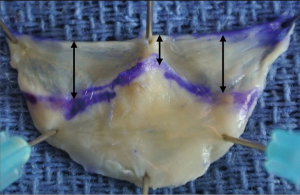
Leaflet surface & coaptation surface
The average leaflet surface area is 3 cm2 in normal TAVs (18,34,35). Leaflet dimensions correlate with body size and also increase with root dilatation. The leaflet surface is divided into two distinct areas: the coaptation surface and the leaflet belly. The coaptation surface is composed of two adjacent areas called the lunule; each lunule extends from the nodulus of arantius to the commissure. The coaptation area composed of the two lunules, has an average surface of 1.2 cm2 (0.6 cm2 per lunule) in the normal TAV, representing approximately 40% of the leaflet surface (Figure 6) (18). Other studies, using three-dimensional imaging, report a coaptation surface between adjacent leaflets (one lunule) of 0.5 cm2 by echocardiography and 0.8 cm2 by CT (33,36). The coaptation surface remains difficult to assess especially intraoperatively; therefore, two-dimensional measurements (e.g., echocardiography) of the coaptation area are preferred to assess the valve, after valve-sparing surgeries or AV-repair procedures. The coaptation height, also called eH is the orthogonal distance from the basal ring to the tip of leaflet at the center of the valve, the Nodulus of Arantius. The average normal eH distance is about 9 to 10 mm in adults with a normal AV configuration (8). eH can be measured intraoperatively with a dedicated caliper (37) and it can be measured by transesophageal echocardiography. Coaptation length is the height of the zone of leaflet apposition (height of the lunule); it is best measured by three-dimensional echocardiography or CT. Coaptation height is on average 3 mm at the level of the Nodulus of Arantius and it increases to 6 mm on average at the middle portion of the lunule (18,33). Coaptation length, as well as eH, can be used as a predictor for repair durability (13,38).
The commissure height
The commissure height or the root height is measured from the basal ring to the STJ. In normal TAVs, the average commissure height is 21 mm by direct measurement of fresh aortic homograft specimen, and 17–18 mm by CT imaging (18,32,33). The commissural height between the right/non-coronary leaflets is slightly larger compared to the height of the commissure between the left/right and the non/left. Thubrikar et al. demonstrated in an echocardiographic study that the commissural height increases from 21 mm to approximately 30 mm in patients with root dilatation (5). This observation has direct implications for valve-sparing surgeries as the resuspension of the commissure inside the graft is crucial for the final configuration of the valve. If commissures are implanted too low, the coaptation area drops towards the ventricle, and accordingly a prolapse is induced. In all techniques of valve-sparing surgery, it is recommended to resuspend the commissures as high as possible. In the remodeling technique, the commissural height corresponds to the length of the slit made in the graft. In valve-sparing reimplantation, using Valsalva-type grafts, sizing of the commissure is of utmost importance as the valve must fit into the sinus portion of the graft from its inflow (basal ring) to the neo-STJ (tip of the commissures). In such grafts, the diameter of the graft (label size) generally corresponds to the height of the sinus portion. The graft size used in the reimplantation technique generally ranges from 28 to 34 mm, with sizes 30 and 32 mm being the most commonly used grafts, which also corresponds to the commissural heights found in patients with root dilatation.
Bicuspid aortic valve geometry
Usefulness of current BAV classifications
Early in the new millennium, the Sievers classification for BAVs was introduced to the clinical community and has since become the most widely utilized classification for BAV to date (39). The Sievers classification generally distinguishes between three types of BAVs. Type 0 is a symmetric BAV without a raphe, and two identical cusps with varying orientations. Type I BAV have one raphe, thus a fusion of two adjacent leaflets. And this fusion can occur with decreasing incidence at the left/right-, right/non-, and non/left-commissures, and are subcategorized as type Ia, Ib, and Ic, respectively. Type II BAV, according to the Sievers classification are valves with two raphae. These valves however, are no longer considered as being part of the BAV spectrum, but represent a different category and are thus called unicuspid AVs. Unicuspid AVs have a very different pathophysiology from BAVs, with AV disease appearing much earlier in life (40,41).
Moreover, roughly 95% of all BAV fall into the Sievers type I classification. In recent years, we have learned that type I valves represent a very wide spectrum of valve phenotypes that range from perfectly symmetric valves as in type 0, with a raphe however, to valves that more closely resemble TAVs, and independent of which two leaflets are fused. Hence, the utility of the Sievers classification remains quite low, especially in the context of BAV repair. Therefore, newer and more comprehensive classifications have thus emerged in recent years (42).
Goal of BAV repair
As previously mentioned, BAVs are a heterogenous group of valves that can be associated with asymmetric sinuses and can present with aortopathies of the root, the ascending aorta, as well as coarctation of the aorta (42). The patients can present with early valve degeneration and valve stenosis, but can also present with AR, which is often associated with annular and root enlargement (43). In BAV-regurgitation, the annulus can reach 30–32 mm in diameter on average (24). As already mentioned, the annular dilation is related to a dilation of the anterior septal or muscular portion of the AVJ. And this is probably not a coincidence, since, as in mitral valve disease, a prolapse develops more frequently in the leaflet supported by the dilated portion of the annulus (i.e., the fused leaflet in BAV). The fused cusp is generally less mobile and smaller in gH than the non-fused lealfet. The sinuses are often asymmetric with a larger fused lealfet sinus and a smaller non-fused lealfet sinus.
In order to repair a regurgitant BAV, it’s important to remodel and stabilize the annulus at all levels (VBR/AVJ, root & STJ = FAA), and to correct a fused lealfet prolapse while respecting the mobility of the fused lealfet. We generally achieve this with our 180°-reimplantation technique, which achieves all these goals and has been previously described in detail (22). Not only does the Reimplantation technique in this setting stabilize the FAA, but it also allows for direct closure of the fused leaflet and preserve its mobility. Effectively, the 180° reimplantion technique, with its’ selective annuloplasty and commissural re-orientation at 180°, reduces the valve orifice area covered by the fused leaflet and increases the area covered by the non-fused leaflet (22,24).
With the selective annuloplasty, the larger fused cusp sinus is reduced to an equal size to the originally smaller not fused sinus. Not only does the more mobile non-fused cusp now cover a relatively larger valve orifice area, but decreasing the fused cusp sinus, allows for direct closure of the line of fusion, preserving fused cusp mobility and obviating the need for pericardial patch extension, which has been associated with valve stenosis later (44). Long-term results with this technique are excellent and have previously been reported to restore normal life expectancy with a 97% 12-year survival and 91% 12-year freedom from AR > grade 2 (14).
Parameter of importance and their relationship
In recent years, our understanding of BAV morphologies and phenotypes has fundamentally improved with newer and more detailed anatomical studies on BAVs (17). In this article, we have uncovered the relationship between commissural orientation, length of lealfet fusion and raphe height, which has set the foundation for a new surgical BAV classification (described below) and has thus substantially improved our understanding of BAV morphologies. We have found that commissural orientation, which is the angle of the coaptation line between the two lealfets measured on the side of the non-fused lealfet (24), is associated with a certain length of lealfet fusion and raphe height.
The commissural orientation can range from 180°–120° (measured on TEE in diastole), and as the valve becomes more asymmetric and the commissural angle becomes smaller, the line of leaflet fusion recedes (becomes shorter) and the raphe height increases closer towards the level of the STJ, and thus closer to the height of a normal commissure (17). In a large population of BAV patients who were candidates for AV repair, the average BAV phenotype has a commissural orientation of 150°, with a length of fusion of 14 mm and a raphe height of 13 mm. The average gH is 23 mm for the non-fused leaflet and only 19 mm for the fused leaflet (17).
A new surgical classification
Based on the aforementioned findings, we were able to introduce a new surgical classification, which better describes the morphologic properties of a wide spectrum of BAVs (Figure 7) (22,24,45). For this purpose, the different BAV phenotypes were subcategorized into symmetric (type A), asymmetric (type B), and very asymmetric (type C) phenotypes, based on commissural orientation. This surgical classification provides the surgeon with a clear picture of the valve in preparation of surgical repair and valve-sparing surgery.
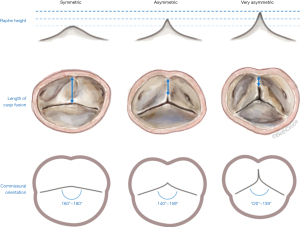
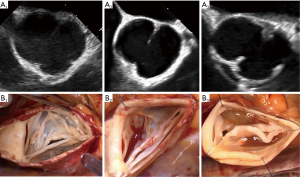
Symmetric BAVs (type A) ranged from 160°–180°, with a long line of fusion and a low raphe. The fusion length equals the gH of the fused and non fused leaflets. The root may present with either two or three sinuses of Valsalva. On a reparability scale, this type is generally considered as “easy” to repair, since the valve is already symmetric with sufficient tissues on both lealfets (Figure 8).
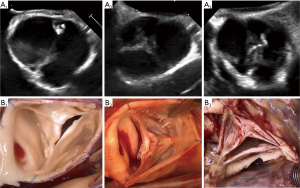
Asymmetric BAVs (type B) ranged from 140°–159°, with a shorter line of fusion, and a slightly higher raphe. The length of fusion is shorter than the gH of the two fused leaflet components. The raphe height approximately reaches half of the root height (Figure 9). On a reparability scale, this type is an “intermediate level”, because the fused leaflets are unequal, retraction can be present on the fused leaflet and valve geometry may need to be modified (symmetrization).
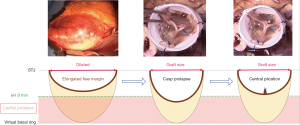
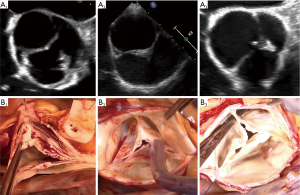
Very asymmetric BAVs (type C) ranged from 120°–139° with a short line of fusion and a high raphe (17). The gH of all leaflets approach the size observed in the TAV or less, and hence the fusion is short and the raphe height extends to almost the level of the STJ. Very asymmetric phenotypes can have additional subcategories, such as the chordal type, and the forme fruste BAV (24). On a reparability scale, this type is considered as “difficult” to repair and requires more experience. Repair of very asymmetric BAVs is less standardized than that for the two other types, and involves more refined techniques, such as patch augmentation and neo-commissure creation (Figure 10) (24).
Conclusions
AV morphologies are complex and diverse, and the anatomic and functional inter-relationships with the aortic root, or FAA, have to be understood in order to perform valve-sparing surgeries and valve repair proficiently. Modifications of the aortic root can lead to dysfunction of the valve, which can easily be remedied if fully understood while applying basic surgical principles. Different valve morphologies, such as BAVs, are not a contraindication to valve-sparing surgery or valve repair, but the unique features of these abnormal phenotypes render the repair more complex and challenging. With the rise of endovascular replacement options, and the now hotly debated best life-time management of AV-disease, it’s important to understand that valve preservation is able to restore normal life-expectancy, with a close to normal quality-of-life. It is therefore important that we educate surgeons on how to perform valve-sparing operations safely. The first step to achieving this, is to understand the functional anatomy of different AV phenotypes and the complex inter-relationships of the AV with the aortic root or FAA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swanson M, Clark RE. Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ Res 1974;35:871-82. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, van Dyck M, et al. Free margin length and geometric height in aortic root dilatation and leaflet prolapse: implications for aortic valve repair surgery. Eur J Cardiothorac Surg 2020;57:124-32. [Crossref] [PubMed]
- Thubrikar MJ, Labrosse MR, Zehr KJ, et al. Aortic root dilatation may alter the dimensions of the valve leaflets. Eur J Cardiothorac Surg 2005;28:850-5. [Crossref] [PubMed]
- Marom G, Haj-Ali R, Rosenfeld M, et al. Aortic root numeric model: annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg 2013;145:406-11.e1. [Crossref] [PubMed]
- Roman MJ, Devereux RB, Kramer-Fox R, et al. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989;64:507-12. [Crossref] [PubMed]
- Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010;38:400-6. [Crossref] [PubMed]
- Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol 2012;110:1189-94. [Crossref] [PubMed]
- Padial LR, Oliver A, Sagie A, et al. Two-dimensional echocardiographic assessment of the progression of aortic root size in 127 patients with chronic aortic regurgitation: role of the supraaortic ridge and relation to the progression of the lesion. Am Heart J 1997;134:814-21. [Crossref] [PubMed]
- Keane MG, Wiegers SE, Plappert T, et al. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000;102:III35-9. [Crossref] [PubMed]
- Hanke T, Charitos EI, Stierle U, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg 2009;137:314-9. [Crossref] [PubMed]
- Kunihara T, Aicher D, Rodionycheva S, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg 2012;143:1389-95. [Crossref] [PubMed]
- de Meester C, Vanovershelde JL, Jahanyar J, et al. Long-term durability of bicuspid aortic valve repair: a comparison of 2 annuloplasty techniques. Eur J Cardiothorac Surg 2021;60:286-94. [Crossref] [PubMed]
- Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg 2017;153:S65-71. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, et al. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg 2016;50:350-60. [Crossref] [PubMed]
- de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019;ezz033. [Crossref] [PubMed]
- De Kerchove L, Momeni M, Aphram G, et al. Free margin length and coaptation surface area in normal tricuspid aortic valve: an anatomical study. Eur J Cardiothorac Surg 2018;53:1040-8. [Crossref] [PubMed]
- Anderson RH, Devine WA, Ho SY, et al. The myth of the aortic annulus: the anatomy of the subaortic outflow tract. Ann Thorac Surg 1991;52:640-6. [Crossref] [PubMed]
- Toh H, Mori S, Tretter JT, et al. Living Anatomy of the Ventricular Myocardial Crescents Supporting the Coronary Aortic Sinuses. Semin Thorac Cardiovasc Surg 2020;32:230-41. [Crossref] [PubMed]
- de Kerchove L, Jashari R, Boodhwani M, et al. Surgical anatomy of the aortic root: implication for valve-sparing reimplantation and aortic valve annuloplasty. J Thorac Cardiovasc Surg 2015;149:425-33. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, El Khoury G. Bicuspid aortic valve repair: the 180°-Reimplantation technique. Ann Cardiothorac Surg 2022;11:473-81. [Crossref] [PubMed]
- Mastrobuoni S, de Kerchove L, Jahanyar J, et al. Commentary: The depth of the virtual basal ring. J Thorac Cardiovasc Surg 2023;165:1344-5. [Crossref] [PubMed]
- Jahanyar J, El Khoury G, de Kerchove L. Commissural geometry and cusp fusion insights to guide bicuspid aortic valve repair. JTCVS Tech 2021;7:83-92. [Crossref] [PubMed]
- Doty DB. Anomalous origin of the left circumflex coronary artery associated with bicuspid aortic valve. J Thorac Cardiovasc Surg 2001;122:842-3. [Crossref] [PubMed]
- Fedak PW, Verma S, David TE, et al. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002;106:900-4. [Crossref] [PubMed]
- Palomo AR, Schrager BR, Chahine RA. Anomalous origin of the right coronary artery from the ascending aorta high above the left posterior sinus of Valsalva of a bicuspid aortic valve. Am Heart J 1985;109:902-4. [Crossref] [PubMed]
- Hechadi J, De Kerchove L, Tamer S, et al. Modified valve-sparing reimplantation technique for para-commissural coronary ostia. Eur J Cardiothorac Surg 2014;45:937-8. [Crossref] [PubMed]
- Schäfers HJ, Schmied W, Marom G, et al. Cusp height in aortic valves. J Thorac Cardiovasc Surg 2013;146:269-74. [Crossref] [PubMed]
- Khelil N, Sleilaty G, Palladino M, et al. Surgical anatomy of the aortic annulus: landmarks for external annuloplasty in aortic valve repair. Ann Thorac Surg 2015;99:1220-6. [Crossref] [PubMed]
- Hagendorff A, Evangelista A, Fehske W, et al. Improvement in the Assessment of Aortic Valve and Aortic Aneurysm Repair by 3-Dimensional Echocardiography. JACC Cardiovasc Imaging 2019;12:2225-44. [Crossref] [PubMed]
- Izawa Y, Mori S, Tretter JT, et al. Normative Aortic Valvar Measurements in Adults Using Cardiac Computed Tomography—A Potential Guide to Further Sophisticate Aortic Valve-Sparing Surgery. Circ J 2021;85:1059-67. [Crossref] [PubMed]
- Jelenc M, Jelenc B, Poglajen G, et al. Aortic valve leaflet and root dimensions in normal tricuspid aortic valves: A computed tomography study. J Card Surg 2022;37:2350-7. [Crossref] [PubMed]
- Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. Am J Cardiol 1985;55:454-61. [Crossref] [PubMed]
- Kunzelman KS, Grande KJ, David TE, et al. Aortic root and valve relationships. Impact on surgical repair. J Thorac Cardiovasc Surg 1994;107:162-70. [Crossref] [PubMed]
- Sohmer B, Hudson C, Atherstone J, et al. Measuring aortic valve coaptation surface area using three-dimensional transesophageal echocardiography. Can J Anaesth 2013;60:24-31. [Crossref] [PubMed]
- Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009;2:931-9. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, Tsai PI, et al. Unicuspid aortic valves are no bicuspid aortic valves-It's time to retire the Sievers-classification. J Card Surg 2022;37:4202-3. [Crossref] [PubMed]
- Aicher D, Schäfers HJ. Bicuspidization of the regurgitant unicuspid aortic valve. Multimed Man Cardiothorac Surg 2010;2010:mmcts.2009.004069.
- Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. J Thorac Cardiovasc Surg 2021;162:e383-414. [Crossref] [PubMed]
- Al-Atassi T, Hynes M, Sohmer B, et al. Aortic root geometry in bicuspid aortic insufficiency versus stenosis: implications for valve repair. Eur J Cardiothorac Surg 2015;47:e151-4. [Crossref] [PubMed]
- Spadaccio C, Nenna A, Henkens A, et al. Predictors of long-term stenosis in bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Jahanyar J, Said SM, Tsai PI. The De Kerchove/El Khoury/Schäfers' classification: a contemporary repair-oriented categorization of bicuspid aortic valves. Eur J Cardiothorac Surg 2023;63:ezad174. [Crossref] [PubMed]






