Minimally invasive surgical coronary artery bypass in women
Introduction
Cardiovascular disease, including coronary artery disease (CAD), remains the leading cause of death for women worldwide (1,2). There is increasing evidence that women with ischemic heart disease demonstrate a different phenotype than men. Diagnostic studies demonstrate sex differences in the functional and anatomical consequences of CAD (3). Furthermore, women experience worse outcomes than men after revascularization; women are at increased risk of mortality after both percutaneous coronary intervention (PCI) (4-6) and surgery (7-15). After coronary artery bypass grafting (CABG), women experience increased morbidity, including major adverse cardiac and cerebrovascular events (MACCE) (11,16), myocardial infarction (MI) (11,16), stroke (8,16), repeat revascularization (11), deep sternal wound infection (8,17), respiratory failure (18) and blood transfusion requirements (19).
Recognition of the unique phenotype of CAD and inferior post-operative outcomes in women demands that we re-think surgical revascularization strategies. Large retrospective studies using propensity matching and multivariable logistic regression suggest that women may derive greater benefit than men from CABG performed without cardiopulmonary bypass, or “off-pump” (20-23). In addition, use of robotic off-pump hybrid coronary revascularization (HCR)—consisting of minimally invasive CABG with PCI—is as safe as conventional on- and off-pump multivessel CABG in women and may further improve perioperative outcomes (24,25). Thus, focus on the appropriate patient selection and operative management of women requiring CABG is key to improving outcomes.
Widespread adoption of minimally invasive CABG has been limited by technical demands and lack of long-term outcome data. Minimally invasive CABG was introduced in the 1990s (26), but left internal mammary artery (LIMA) harvest necessitated extensive rib spreading resulting in increased postoperative pain (27). Robotic mammary harvest, first described in 1999 by Mohr et al. (28) and Loulmet et al. (29), enables LIMA harvest via three robotic ports and facilitates a reduction in size of the anterior thoracotomy incision. Totally endoscopic coronary artery bypass (TECAB) surgery and multivessel revascularization using the bilateral internal mammary arteries are further advancements in technique, but remain limited to high-volume specialized centers (30,31).
A common strategy for minimally invasive CABG involves revascularization of the left anterior descending (LAD) artery without cardiopulmonary bypass through a small left anterior thoracotomy incision with the LIMA, which is harvested with robot assistance. This technique is best suited for patients with isolated proximal LAD stenosis or patients with multivessel CAD undergoing a hybrid approach with PCI (32-34). In the following section, we describe the operative technique for robot-assisted minimally invasive CABG in women requiring coronary revascularization of the LAD. We focus on specific differences in operative planning and technique for performing minimally invasive CABG in women to optimize outcomes in this patient population.
Operative technique
Patient selection/preoperative evaluation
As revascularization is limited to LIMA to LAD bypass grafting only, patients must meet standard indications for single-vessel CABG or hybrid approach. Contraindications to minimally invasive CABG include hemodynamic instability, poor LAD target for off-pump CABG [intramyocardial LAD without segment amenable to anastomosis, chronic total occlusion (CTO) without distal reconstitution] and poor LIMA conduit (i.e., significant subclavian stenosis resulting in diminutive LIMA). Preoperative assessment includes pulmonary function tests and computed tomography (CT) of the chest. Pulmonary function tests are appropriate for patients with known pulmonary disease (e.g., chronic obstructive pulmonary disease) due to the use of single-lung ventilation during the procedure. CT chest angiography can demonstrate critical anatomy for operative planning and evaluation of potential anatomic contraindications (see “Caveats” below).
Preparation
Intubation with a dual-lumen endotracheal tube facilitates the best exposure with left lung isolation; the bronchial blocker is an alternative. If the patient does not tolerate single lung ventilation, then low tidal volume ventilation is an option. External defibrillator pads are required because internal defibrillators will not easily fit through the small incisions. Communication between the anesthesia and surgical teams regarding hemodynamic parameters is critical, particularly during manipulation of the heart. Off-pump anesthesia management principles, including pre-load optimization, are paramount. Emergent cannulation strategy should be anticipated in higher-risk patients in case of hemodynamic instability or ventricular arrhythmia. Prophylactic intra-aortic balloon pump should be considered in patients with reduced ejection fraction.
Patient positioning
The patient is placed on the operating table in supine position with the arms tucked. The sternum and bilateral groins are included in the operative field in case of emergent sternotomy or groin cannulation, respectively. For women with pendulous breasts, antimicrobial incise drapes (Ioban®, 3M Company, Saint Paul, MN, USA) can be utilized to retract the breasts cephalad and medially.
Operation
Robotic port placement
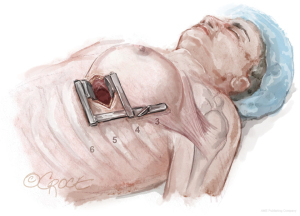
The LIMA is harvested and the pericardium is opened with the da Vinci™ robot (Si and Xi, Intuitive Surgical, Sunnyvale, CA, USA) via ports typically located in the 2nd, 5th, and 7th intercostal spaces (Figure 1). To facilitate port placement, the left lung is isolated, and a 5-mm transparent tip optical trocar and 5-mm camera are placed in the 2nd or 3rd intercostal space at the mid-clavicular line. CO2 is insufflated at a starting pressure of 8 mmHg, with careful monitoring for hemodynamic instability as a tension pneumothorax is created. The insufflation pressure can be increased to 10 to 12 mmHg as tolerated to improve visibility in obese patients. The superior port, or “right hand”, is exchanged for a robot port.
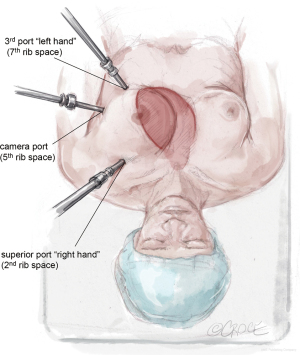
The next robotic port is placed in the 5th intercostal space at the anterior axillary line as the robot “camera port”. In women, this may correlate with breast tissue. To avoid the breast tissue, we make the skin incision lateral to the breast, insert the port into the soft tissue, then frameshift the port anteriorly on the chest wall so that the port enters the chest wall at the anterior axillary line. Inserting the port at a more anterior (versus lateral) location on the chest wall keeps the working space anterior to the heart. Placing the camera port at the mid-axillary line, or more posterior, risks damage to the pericardium and heart.
The third port, or “left hand”, is placed at the midclavicular line just above the diaphragm, which usually correlates to the 7th rib space. Each port should be separated by at least two rib spaces to minimize collisions.
The location of robotic port placement may require adjustment based on the patient’s body habitus, taking into consideration external landmarks and other anatomic concerns identified on preoperative imaging such as CT scan. Optimal port placement is a priority in women due to their relatively smaller thoracic cavity size compared to men, which increases risk of port collision. An advantage of the da Vinci™ Xi robot compared to the Si is a lower likelihood of robotic arm collision. Finally, a nerve block is performed by injecting long-acting local anesthesia into the intercostal spaces during port placement.
Robot-assisted LIMA harvest
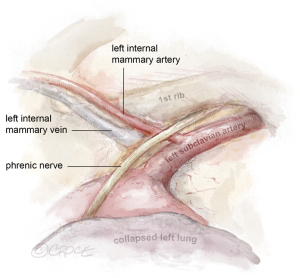
The daVinci™ robot is docked and key landmarks are identified: left subclavian artery, LIMA, left internal mammary vein, and phrenic nerve (Figure 2). The subclavian vein is usually visualized later in LIMA dissection. The robotic instruments include a microbipolar grasper in the left hand and a spatula in the right hand. Cautery settings are based on surgeon preference, but a common setting for the spatula is level 3 on the Xi. Due to lack of haptic feedback, adequate visualization is paramount. First, the pericardial fat is removed off the pericardium in the cranial to caudal direction to improve working space between the pericardium and the chest wall. Next, the pleural fat overlying the LIMA is dissected off the endothoracic fascia until the LIMA is visible.
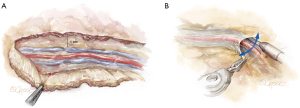
The LIMA is most visible at the mid-thoracic location, which is the optimal starting point. The endothoracic fascia is incised using electrocautery approximately 1 cm lateral to the LIMA (Figure 3A). The fascia is mobilized off the LIMA pedicle using a sweeping motion with the spatula (Figure 3B). In contrast to the standard LIMA harvest with sternotomy, which is conducted medially to laterally, robotic LIMA harvest is performed with a lateral to medial dissection. It is our preference to remove the fascia from the LIMA but leave the mammary veins attached, which maximizes length while minimizing manipulation of the LIMA vessel. The LIMA is first harvested in the cranial direction up to the subclavian vein, and then in the caudal direction past the mammary bifurcation, which are the standard anatomic reference points.
Electrocautery is used for division of side branches. Rarely, clips are utilized for large branches, which requires instrument exchange. The dissection is optimized with smooth blunt dissection alternating with deliberate electrocautery use. The fat surrounding the vessels can be swept toward or away from the LIMA pedicle, but dissection through the fat should be avoided to prevent bleeding and loss of tissue planes. The transversalis fascia must be mobilized off the chest wall to improve visualization of the distal LIMA. The transversalis fascia starts at the mid-LIMA, as opposed to the most inferior aspect of the LIMA pedicle when harvested via sternotomy. The LIMA bifurcation correlates with the costal margin. Importantly, care should be taken not to mistake large side branches, particularly at the level of the 5th intercostal space, with the true bifurcation. Intravenous heparin (5,000 units) is administered immediately prior to ligation of the LIMA. The LIMA is clipped distally with robot medium Hemolock clips (Teleflex, Wayne, PA, USA) and divided with robot Potts scissors (Intuitive Surgical).
Opening the pericardium and identification of the LAD
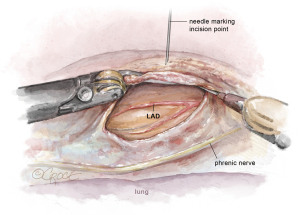
Attention is then turned to the pericardium. The phrenic nerve is identified posteriorly. The pericardium is opened anteriorly with electrocautery in a similar fashion to the open technique. The pericardial incision is continued longitudinally from the pulmonary artery to the diaphragm, thereby exposing the LAD (Figure 4). With the pericardium open, the precise intrathoracic location of the LAD is determined, and a needle can be inserted through the chest wall by the bedside surgeon to correlate the skin incision with the LAD. The location is then marked on the skin. Finally, a pericardial key-hole can be made posterior to the phrenic nerve to allow fluid to drain from the pericardial well during the CABG portion of the procedure.
The robot is undocked and a chest tube in placed through the inferior-most port. The chest tube is placed on suction for remainder of procedure to optimize exposure.
Anterior thoracotomy
The LIMA to LAD anastomosis is performed through a 6-cm left anterior thoracotomy incision that enables direct visualization of the heart and LAD (Figure 5). For men, the incision can correlate with the location marked on the skin, corresponding to the LAD, usually the 4th or 5th intercostal space. For women, management of the breast tissue is key when performing the anterior thoracotomy to avoid risk of wound dehiscence and infection (Figure 6). The skin incision is made approximately 0.5–1 cm offset from the inframammary line and into the breast to avoid the inframammary crease. Dissection is continued through the soft tissue straight down to the chest wall and the breast is mobilized off the chest wall until the inferior edge of the pectoralis muscle is reached. By creating a breast flap, the location for entry into thoracic cavity will be offset from the location of initial skin incision.
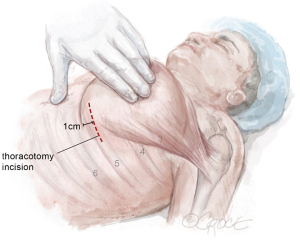
Next, the pectoralis muscle is elevated off the chest wall to expose the ribs. The intercostal space (usually the 4th or 5th) is entered. The appropriate intercostal space depends on the size of the heart and location of the LAD target identified on LHC, preoperative CT chest, and intraoperative localization with the robot. The intercostal space is opened widely from the sternum to the lateral chest wall to optimize rib spreading.
LIMA to LAD anastomosis
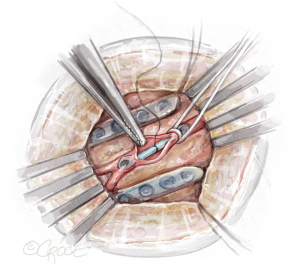
A small retractor is placed in the thoracotomy. A pericardial well is created with sutures placed in the medial and lateral aspects of the pericardium. If the LAD is located laterally, sequential pericardial stitches may be placed further laterally bringing the LAD into a more medial position. Visual identification of the LAD is then confirmed with four steps: (I) an index finger palpates the entire length of the LAD and the apex is confirmed; (II) the LAD is compressed and septal bowing is confirmed on the 4-chamber transesophageal echo (TEE) view; (III) the right ventricle is compressed and free wall bowing is confirmed on the 4-chamber TEE view; and (IV) the diagonal coronary arteries are visualized (35).
Next, a tissue stabilizer device (Octopus® Nuvo, Medtronic, Minneapolis, MN, USA) is placed on the LAD (Figure 7). The suction foot pad is held in place with a stabilizer bar passed through a subcostal incision. A left subcostal stab incision is made, and a tonsil creates a tunnel through the diaphragm into the thoracic cavity. The stabilizer bar is then placed through the subcostal incision, through the diaphragm, and over the pericardium to connect to the foot pad. The surgeon’s free hand can be placed into the chest through the thoracotomy incision to guide the stabilizer safely into the thoracic cavity, avoiding inadvertent injury to intrathoracic structures including the heart. The stabilizer rod is secured to a side bar attached to the bed rail. With the stabilizer secured, the hemodynamics are reviewed to assess for left ventricular outflow tract obstruction and tolerance of the stabilizer.

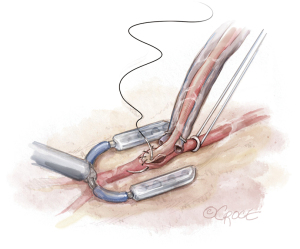
The LAD is occluded, and an intracoronary shunt is placed (Figure 8). At least 1 cm of the LAD is cleared off. A silastic retractor tape with a blunt needle is passed behind the LAD twice, which facilitates occlusion of the LAD proximal to the planned anastomotic site. If there is significant collateralization to the LAD, then distal occlusion may also be necessary. The arteriotomy is performed with the retractor tape open, and the intracoronary shunt is placed. The retractor tape is tightened to occlude the LAD around the shunt. A hand-sewn LIMA to LAD anastomosis is performed under direct vision in a standard fashion (Figure 9). The shunt is removed near completion of the anastomosis.
Completion
After completion of the LIMA-LAD anastomosis, graft patency and blood flow are evaluated using a flow probe (Medistim flow probe, Oslo, Norway) with the retractor tape still in place to rule out competitive flow. Blood flow is visualized with Doppler ultrasound (Medistim high-frequency ultrasound, Oslow, Norway). Once adequate LIMA flow is confirmed, the retractor tape is removed from the proximal LAD and native coronary flow is reestablished. A piece of pericardial fat is draped loosely over the anastomosis to secure the LIMA in position and create a barrier between the chest wall and the anastomosis. The heparin is reversed with protamine. Exquisite care must be taken to identify any bleeding site. If needed, a camera can be placed through the thoracotomy incision to check for bleeding from the robot port sites. The thoracic cavity should be thoroughly irrigated until the fluid is clear. The lung is re-inflated under direct visualization, and the LIMA is inspected to ensure that the graft position is maintained once the lung has been fully reinflated (Figure 10). The ribs, pectoral muscle, soft tissue, and skin, including the robot port sites, are closed. Patients are often extubated in the operating room, enabling earlier transfer out of the intensive care unit and discharge home by post-operative day three or four.
Comments
Clinical results
Minimally invasive CABG is safe and effective in the treatment of proximal LAD stenosis (36-43). Early postoperative mortality (in-hospital/30-day) is low, with most recent studies reporting rates of ≤1% (39-41,43-48). Minimally invasive CABG is associated with a ten-year survival of 76–90% (40,41,43,44,47) and freedom from MACCE of 70–85% (40,43,44). The incidence of target vessel reintervention following minimally invasive CABG is ≤5% (37,42-44,47,49,50), which is significantly lower than PCI for isolated LAD disease (37,42,45,49,50).
Overall, minimally invasive CABG has shown promising short-term results with several series reporting reduced surgical morbidity and patient recovery times as well as higher quality of life scores compared to conventional sternotomy CABG (40,43,46,51-55). Reported rates of perioperative complications following minimally invasive CABG are comparable or lower than conventional sternotomy CABG (38,43,48), and risk of conversion to sternotomy is ≤2% (38,41,44,47)
Advantages
Minimally invasive CABG with the LIMA to LAD offers the durable graft patency of the LIMA over PCI while avoiding sternotomy and cardiopulmonary bypass (56). Use of a sternal-sparing approach eliminates the risk of sternal wound infections and improves cosmesis, which may be of particular importance in women given the increased risk of deep sternal wound infections in this population (8,17). Avoidance of cardiopulmonary bypass may further reduce the risk of respiratory complications and blood transfusion requirements (57), both of which have been shown to occur with greater frequency in women compared to men after CABG (18,19).
Although there are no studies that demonstrate a modifying effect of minimally invasive technique on sex differences in survival after CABG, a minimally invasive approach may impact operative time and morbidity (41,58,59). Although data is limited, in retrospective reviews with propensity score matching and multivariate regression analysis, women and men who undergo minimally invasive CABG experience similar in-hospital and long-term mortality (41,58,59). However, women experience longer operative times, increased wound healing complications, and increased rate of blood transfusions (58,59). Additional data from large, prospective studies is necessary to further investigate the specific impact of minimally invasive techniques on outcomes in women who undergo CABG.
Caveats
Minimally invasive CABG, particularly with robot assistance, involves a steep learning curve for the operating surgeon as well as the operating room team. Case volume can affect outcomes following minimally invasive CABG (60). Despite its established safety and feasibility in appropriately selected patients, widespread implementation of minimally invasive CABG has yet to occur, and application of the procedure continues to be limited to a small number of experienced centers (30,31). Appropriate patient selection is critical to achieving acceptable outcomes and is aided with focused preoperative imaging (i.e., CT chest with angiography) and multidisciplinary collaboration.
To optimize patient selection and operative planning, we routinely perform preoperative CT angiography (CTA) of the chest in patients undergoing consideration for minimally invasive CABG. In our experience, CTA provides critical information related to characterization of the LAD, including intrathoracic location, presence of intramyocardial course, and thickness of epicardial fat surrounding the LAD. The presence of an intramyocardial segment, CTO, or excess epicardial fat overlying the LAD may be prohibitive for performing minimally invasive CABG due to increased risk of conversion to sternotomy or complicated anastomosis.
Preoperative CTA also allows for evaluation of graft quality, thoracic cavity dimensions, underlying lung disease, and emergent cardiopulmonary bypass strategies. CTA can demonstrate LIMA patency and presence of subclavian stenosis that may result in diminutive LIMA flow. Skeletal abnormalities affecting the thoracic cavity (i.e., scoliosis, pectus excavatum/carinatum) can limit robot mobility and may affect patient candidacy for the procedure. Preoperative CT will also reveal underlying lung disease. The addition of CT abdomen/pelvis will demonstrate the presence and extent of peripheral vascular disease, which can affect emergent cardiopulmonary bypass strategies. Importantly, although non-contrast CT is limited in resolution, it may still be useful in operative planning and the evaluation of potential contraindications to a minimally invasive approach described above.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. [Crossref] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139-596. [Crossref] [PubMed]
- Rodriguez Lozano PF, Rrapo Kaso E, Bourque JM, et al. Cardiovascular Imaging for Ischemic Heart Disease in Women: Time for a Paradigm Shift. JACC Cardiovasc Imaging 2022;15:1488-501. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Cao D, et al. Sex-Related Outcomes of Medical, Percutaneous, and Surgical Interventions for Coronary Artery Disease: JACC Focus Seminar 3/7. J Am Coll Cardiol 2022;79:1407-25. [Crossref] [PubMed]
- Kosmidou I, Leon MB, Zhang Y, et al. Long-Term Outcomes in Women and Men Following Percutaneous Coronary Intervention. J Am Coll Cardiol 2020;75:1631-40. [Crossref] [PubMed]
- Gul B, Shah T, Head SJ, et al. Revascularization Options for Females With Multivessel Coronary Artery Disease: A Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv 2020;13:1009-10. [Crossref] [PubMed]
- Angraal S, Khera R, Wang Y, et al. Sex and Race Differences in the Utilization and Outcomes of Coronary Artery Bypass Grafting Among Medicare Beneficiaries, 1999-2014. J Am Heart Assoc 2018;7:e009014. [Crossref] [PubMed]
- Gaudino M, Chadow D, Rahouma M, et al. Operative Outcomes of Women Undergoing Coronary Artery Bypass Surgery in the US, 2011 to 2020. JAMA Surg 2023;158:494-502. [Crossref] [PubMed]
- Mohamed W, Mohamed MO, Hirji S, et al. Trends in sex-based differences in outcomes following coronary artery bypass grafting in the United States between 2004 and 2015. Int J Cardiol 2020;320:42-8. [Crossref] [PubMed]
- Mahowald MK, Alqahtani F, Alkhouli M. Comparison of Outcomes of Coronary Revascularization for Acute Myocardial Infarction in Men Versus Women. Am J Cardiol 2020;132:1-7. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Alexander JH, et al. Sex differences in outcomes after coronary artery bypass grafting: a pooled analysis of individual patient data. Eur Heart J 2021;43:18-28. [Crossref] [PubMed]
- Blankstein R, Ward RP, Arnsdorf M, et al. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 Midwestern hospitals. Circulation 2005;112:I323-7. [Crossref] [PubMed]
- Swaminathan RV, Feldman DN, Pashun RA, et al. Gender Differences in In-Hospital Outcomes After Coronary Artery Bypass Grafting. Am J Cardiol 2016;118:362-8. [Crossref] [PubMed]
- Vaccarino V, Abramson JL, Veledar E, et al. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation 2002;105:1176-81. [Crossref] [PubMed]
- Lawton JS. Sex and gender differences in coronary artery disease. Semin Thorac Cardiovasc Surg 2011;23:126-30. [Crossref] [PubMed]
- Bryce Robinson N, Naik A, Rahouma M, et al. Sex differences in outcomes following coronary artery bypass grafting: a meta-analysis. Interact Cardiovasc Thorac Surg 2021;33:841-7. [Crossref] [PubMed]
- Toumpoulis IK, Anagnostopoulos CE, Balaram SK, et al. Assessment of independent predictors for long-term mortality between women and men after coronary artery bypass grafting: are women different from men? J Thorac Cardiovasc Surg 2006;131:343-51. [Crossref] [PubMed]
- Matyal R, Qureshi NQ, Mufarrih SH, et al. Update: Gender differences in CABG outcomes-Have we bridged the gap? PLoS One 2021;16:e0255170. [Crossref] [PubMed]
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. [Crossref] [PubMed]
- Ter Woorst JF, Hoff AHT, Haanschoten MC, et al. Do women benefit more than men from off-pump coronary artery bypass grafting? Neth Heart J 2019;27:629-35. [Crossref] [PubMed]
- Puskas JD, Kilgo PD, Lattouf OM, et al. Off-pump coronary bypass provides reduced mortality and morbidity and equivalent 10-year survival. Ann Thorac Surg 2008;86:1139-46; discussion 1146. [Crossref] [PubMed]
- Puskas JD, Kilgo PD, Kutner M, et al. Off-pump techniques disproportionately benefit women and narrow the gender disparity in outcomes after coronary artery bypass surgery. Circulation 2007;116:I192-9. [Crossref] [PubMed]
- Mack MJ, Brown P, Houser F, et al. On-pump versus off-pump coronary artery bypass surgery in a matched sample of women: a comparison of outcomes. Circulation 2004;110:II1-6. [Crossref] [PubMed]
- Torregrossa G, Sá MP, Van den Eynde J, et al. Hybrid robotic off-pump versus conventional on-pump and off-pump coronary artery bypass graft surgery in women. J Card Surg 2022;37:895-905. [Crossref] [PubMed]
- Torregrossa G, Sá MP, Van den Eynde J, et al. Robotic hybrid coronary revascularization versus conventional off-pump coronary bypass surgery in women with two-vessel disease. J Card Surg 2022;37:501-11. [Crossref] [PubMed]
- Calafiore AM, Di Giammarco G, Teodori G, et al. Midterm results after minimally invasive coronary surgery (LAST operation). J Thorac Cardiovasc Surg 1998;115:763-71. [Crossref] [PubMed]
- Lichtenberg A, Hagl C, Harringer W, et al. Effects of minimal invasive coronary artery bypass on pulmonary function and postoperative pain. Ann Thorac Surg 2000;70:461-5. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999;117:1212-4. [Crossref] [PubMed]
- Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- Une D, Sakaguchi T. Initiation and modification of minimally invasive coronary artery bypass grafting. Gen Thorac Cardiovasc Surg 2019;67:349-54. [Crossref] [PubMed]
- Fatehi Hassanabad A, Kang J, Maitland A, et al. Review of Contemporary Techniques for Minimally Invasive Coronary Revascularization. Innovations (Phila) 2021;16:231-43. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Toeg H, Al-Atassi T, Labinaz M, et al. Hybrid approach for coronary artery revascularization: where do we stand? Curr Opin Cardiol 2014;29:534-41. [Crossref] [PubMed]
- Gąsior M, Zembala MO, Tajstra M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1277-83. [Crossref] [PubMed]
- Atari O, Krishnan S, Bashir M. Identifying the Left Anterior Descending Artery Target in Minimally Invasive CABG. CTSNet 2018; [Crossref]
- Thiele H, Neumann-Schniedewind P, Jacobs S, et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol 2009;53:2324-31. [Crossref] [PubMed]
- Raja SG, Uzzaman M, Garg S, et al. Comparison of minimally invasive direct coronary artery bypass and drug-eluting stents for management of isolated left anterior descending artery disease: a systematic review and meta-analysis of 7,710 patients. Ann Cardiothorac Surg 2018;7:567-76. [Crossref] [PubMed]
- Raja SG, Garg S, Rochon M, et al. Short-term clinical outcomes and long-term survival of minimally invasive direct coronary artery bypass grafting. Ann Cardiothorac Surg 2018;7:621-7. [Crossref] [PubMed]
- Giambruno V, Chu MW, Fox S, et al. Robotic-assisted coronary artery bypass surgery: an 18-year single-centre experience. Int J Med Robot 2018;14:e1891. [Crossref] [PubMed]
- Repossini A, Di Bacco L, Nicoli F, et al. Minimally invasive coronary artery bypass: Twenty-year experience. J Thorac Cardiovasc Surg 2019;158:127-138.e1. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Bergien L, et al. Twenty-year outcomes of minimally invasive direct coronary artery bypass surgery: The Leipzig experience. J Thorac Cardiovasc Surg 2023;165:115-127.e4. [Crossref] [PubMed]
- Patel NC, Hemli JM, Seetharam K, et al. Minimally invasive coronary bypass versus percutaneous coronary intervention for isolated complex stenosis of the left anterior descending coronary artery. J Thorac Cardiovasc Surg 2022;163:1839-1846.e1. [Crossref] [PubMed]
- Mastroiacovo G, Manganiello S, Pirola S, et al. Very Long-term Outcome of Minimally Invasive Direct Coronary Artery Bypass. Ann Thorac Surg 2021;111:845-52. [Crossref] [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single-center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [Crossref] [PubMed]
- Benedetto U, Raja SG, Soliman RF, et al. Minimally invasive direct coronary artery bypass improves late survival compared with drug-eluting stents in isolated proximal left anterior descending artery disease: a 10-year follow-up, single-center, propensity score analysis. J Thorac Cardiovasc Surg 2014;148:1316-22. [Crossref] [PubMed]
- Stanislawski R, Aboul-Hassan SS, Marczak J, et al. Early and long-term clinical outcomes after minimally invasive direct coronary artery bypass grafting versus off-pump coronary surgery via sternotomy in isolated proximal left anterior descending artery disease: A propensity score matching analysis. J Card Surg 2020;35:3412-9. [Crossref] [PubMed]
- Manuel L, Fong LS, Betts K, et al. LIMA to LAD grafting returns patient survival to age-matched population: 20-year outcomes of MIDCAB surgery. Interact Cardiovasc Thorac Surg 2022;35:ivac243. [Crossref] [PubMed]
- Raja SG, Benedetto U, Alkizwini E, et al. Propensity Score Adjusted Comparison of MIDCAB Versus Full Sternotomy Left Anterior Descending Artery Revascularization. Innovations (Phila) 2015;10:174-8. [Crossref] [PubMed]
- Li S, Zhang H, Xiao C, et al. Robotically assisted coronary artery bypass graft surgery versus drug-eluting stents for patients with stable isolated proximal left anterior descending disease. J Card Surg 2021;36:1864-71. [Crossref] [PubMed]
- Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc Interv 2015;8:30-8. [Crossref] [PubMed]
- Trejos AL, Ross I, Scalesse C, et al. Preoperative evaluation of patient anatomy to increase success of robotics-assisted bypass surgery. Innovations (Phila) 2010;5:335-40. [Crossref] [PubMed]
- Leyvi G, Forest SJ, Srinivas VS, et al. Robotic coronary artery bypass grafting decreases 30-day complication rate, length of stay, and acute care facility discharge rate compared with conventional surgery. Innovations (Phila) 2014;9:361-7; discussion 367. [Crossref] [PubMed]
- Bonaros N, Schachner T, Wiedemann D, et al. Quality of life improvement after robotically assisted coronary artery bypass grafting. Cardiology 2009;114:59-66. [Crossref] [PubMed]
- Ruel M, Une D, Bonatti J, et al. Minimally invasive coronary artery bypass grafting: is it time for the robot? Curr Opin Cardiol 2013;28:639-45. [Crossref] [PubMed]
- Lapierre H, Chan V, Sohmer B, et al. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump: a case-matched study. Eur J Cardiothorac Surg 2011;40:804-10. [Crossref] [PubMed]
- Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts--effects on survival over a 15-year period. N Engl J Med 1996;334:216-9. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489-97. [Crossref] [PubMed]
- Gofus J, Vobornik M, Sorm Z, et al. Female sex as a risk factor in minimally invasive direct coronary artery bypass grafting. Scand Cardiovasc J 2019;53:141-7. [Crossref] [PubMed]
- Friedrich C, Berndt R, Haneya A, et al. Sex-specific outcome after minimally invasive direct coronary artery bypass for single-vessel disease. Interact Cardiovasc Thorac Surg 2020;30:380-7. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Walther T, et al. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg 2007;134:663-9. [Crossref] [PubMed]






