Deep hypothermic circulatory arrest
Introduction
Cerebral protection has been the cornerstone of successful aortic arch surgery for almost 40 years. Aneurysms of the aortic arch are among the most challenging cases for surgical treatment and require procedural expertise for their safe conduct. The enduring debate regarding which of the three cerebral protection techniques - straight deep hypothermic circulatory arrest (DHCA), antegrade cerebral perfusion (ACP), and retrograde cerebral perfusion (RCP) - is superior has split the cardiothoracic surgical community into three camps due to personal and institutional preferences, and each camp can provide multiple studies supporting their point of view (1-3).
At our Aortic Institute at Yale, straight DHCA has been the method of choice for the absolute majority of complex procedures involving the aortic arch. We believe that straight DHCA is an especially appealing method of cerebral protection because of its simplicity and effectiveness. In this article, we will provide evidence that straight DHCA is an effective method of brain protection during aortic surgery without any adjunctive cerebral perfusion. We will describe our experience, demonstrate the excellent clinical results achieved with DHCA, and compare our results favorably to published series applying ACP or RCP.
Development of DHCA as a method of cerebral protection - historical aspects
Hypothermia has been known for its organ-preservation properties since the Hippocratic era. In the mid-20th century it was introduced into surgical practice for operations on the brain and heart, providing both safety in stopping circulation as well as an appealing bloodless operative field. In 1952, well before the era of the heart-lung machine, Dr. John Lewis performed arguably the world’s first successful open-heart procedure, using hypothermia to close a secundum-type atrial septal defect (4). This cardinal achievement laid the groundwork for treatment of various congenital heart defects using this technique. However, it quickly became apparent that hypothermia would not suffice for treating more complex congenital defects; thus, gradually interest in this technique waned in North America.
In the 1960s, a young, intelligent, and creative Soviet cardiac surgeon - Professor Eugene N. Meshalkin, who worked in the city Novosibirsk, in central Siberia - started using hypothermia to make possible the treatment of ventricular septal defect and atrioventricular canal. It is reported that he even approached tetralogy of Fallot and implanted prosthetic mitral and aortic valves under intervals of arrest with hypothermia (5). Snow and ice, abundant in Siberia, were used for induction of hypothermia in patients, many of whom were children. This technique of nonperfusion topical hypothermia (28-29 °C) was technically much simpler than cardiopulmonary bypass (CPB), provided enough safe operative time, and did not cause coagulopathy in the postoperative period (6). However, due to Meshalkin’s prejudice against CPB, which at the time was still frankly experimental, he could not successfully approach more complex cardiac pathologies.
Hypothermia was not applied to the treatment of aortic aneurysms for a long time. In 1955, when Cooley and associates performed the first successful aortic arch replacement with a prosthetic graft, they relied on CPB with ascending-to-descending shunting and side arms to the carotid arteries for cerebral protection (7). However this technique was not sufficient to prevent cerebral ischemia and the patient died on the 6th postoperative day. It was not until the mid-1970s that Griepp published the first experience applying hypothermia as a means of cerebral protection to facilitate replacement of the aortic arch (8). Since then, this technique gained popularity worldwide, and many centers in North America, Europe and Asia began utilizing this simple technique to protect the central nervous system during complex operations on the aorta and aortic arch.
In recent years, many centers that perform aortic surgery have (somewhat undeservedly) abandoned the technique of straight hypothermia as a cerebral protection method, in favor of techniques that provide adjunctive perfusion to the brain (ACP, RCP).
Mechanisms underlying hypothermic neuroprotection
Although the brain accounts for only approximately 2% of the body weight, it utilizes 20% of the resting total body oxygen consumption and receives almost 15-20% of the total circulating blood volume from the heart (9). This is due to the fact that the brain’s metabolic rate of oxygen and glucose consumption is multiple times faster than other human organs. Oxygen-dependent glucose metabolism produces ATP, which is the main source of intracellular energy for neurons. Unlike liver or muscle tissues, the brain does not have “storage” for glucose, thus a shortage of its delivery immediately impairs the neuronal function. Changes in levels of oxygen and/or glucose delivery can be compensated by appropriate changes of the blood flow, a phenomenon known as “autoregulation of cerebral flow” (10).
In order to understand the mechanisms by which hypothermia provides neuroprotection, it is important to understand the two main pathways of ischemic neural injury (note that in reality all these processes are closely interrelated and not independent of each other):
(I) When there is lack of oxygen, ATP is synthesized through anaerobic glycolysis, which is not sufficient to maintain normal neuronal function. Concurrently, lactate accumulates in the neurons, lowering the intracellular pH. Such energy depletion and waste product accumulation within brain cells leads quickly to permanent damage and necrosis (11,12).
(II) Calcium ion plays a central role in ischemic neuronal injury. Hypoxia leads to a release of excitatory neurotransmitters, such as glutamate, which in turn activates the N-methyl-D-aspartate (NMDA) channels. Once these channels are activated, calcium ions easily enter the cells and accumulate. Such imbalance in the calcium level leads to activation of intracellular proteases and mitochondrial dysfunction, which result in neuronal cell death (12).
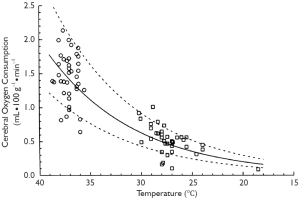
Hypothermia inhibits both of these injury-inducing pathways. It is well established that hypothermia significantly decreases the global cerebral metabolic rate for glucose and oxygen (11). In fact, for every one degree Celsius drop in body temperature, cellular metabolism slows down by an average of 5-7% (11,13). Thus hypothermia actually decreases the demand of the brain cells for oxygen. Simple mathematical calculations, as well as experimental studies, show that at 18 °C the metabolic rate of the human body is only 12% to 25% of the metabolic rate at normal temperature (see Figure 1). The lower the rate of anaerobic metabolism, the less lactate is accumulated and the less pronounced is any cellular acidosis. Lowering the temperature has been proven to reduce to a larger extent ATP breakdown than its synthesis in the brain, which increases cerebral ATP supply for energy-consuming processes (11).
At the same time, hypothermia significantly reduces temperature-dependent release and extracellular levels of excitatory neurotransmitters such as glutamate, an NMDA receptor agonist. An important factor in activation of NMDA receptors is interaction with glycine, the levels of which are depleted in the brain during hypothermic conditions (12). Hence this is a dual mechanism for decreasing the activity of the NMDA channels, which significantly reduces the amount of calcium that is drawn into the neuronal cells. This provides a very effective neuroprotective effect, preventing irreversible neuronal injury.
Other protective mechanisms of hypothermia have been studied and proven, among which are important effects such as inhibition of the pro-apoptotic activity and reduction of free radicals and inflammatory cytokines (12).
Clinical technique of straight DHCA: the Yale experience
Since 1987, straight DHCA has been applied (almost without exception) as the sole means of cerebral protection at our institution. Femoral arterial cannulation is used for perfusion unless the patient is shown (by intraoperative transesophageal echo or preoperative computed tomography) to have arteriosclerotic disease of the descending aorta. In case of arteriosclerosis, we employ either axillary cannulation or direct cannulation of the aneurysm or distal aorta. Venous return is achieved via the right atrial appendage with a two-stage cannula, or in rare cases, via the femoral vein. Carbon dioxide flooding of the field is used in all cases. The extent of aortic resection is determined by the extent of the disease. Typically DHCA is required for partial arch replacement (hemiarch) requiring an open distal anastomosis, and for total arch replacement. As soon as CPB is initiated, the patient is cooled down to 19 °C (for hemiarch) or to 18 °C (for total arch). Cooling takes from 30 to 40 minutes, depending on the size of the patient. Temperature monitoring is conducted solely via a probe in the urinary bladder. The head is packed in ice to achieve topical cooling. Steroids are routinely administered for all patients before CPB is initiated and alpha-stat management is used for acid-base balance. After termination of DHCA, the rewarming usually takes about 60 minutes. We prefer gentle rewarming (gradient between blood and bath temperature less than 10 °C) in order to prevent potential protein denaturation (15,16).
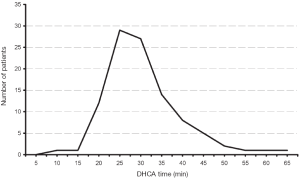
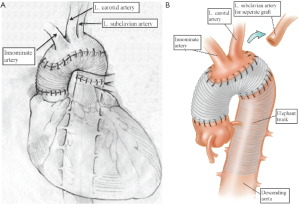
Our extensive 25-year experience of conducting DHCA during complex procedures on the aortic arch has shown that the absolute majority of ascending and hemiarch cases can be safely achieved within a time frame of less than 40 minutes of DHCA. In our recent study, we evaluated the results of DHCA application in 394 patients, which showed that the mean DHCA time was 31 minutes, ranging from 10 to 66 minutes (Figure 2) (15). Total arch replacement can also be performed within 20-40 minutes for the distal anastomosis and 20 minutes for the arch anastomoses. We favor a specific technique of aortic arch reconstruction, which is time expedient: instead of creating a three-vessel Carrel patch, we prefer to use a two-vessel island with just the innominate and left common carotid arteries (Figure 3). This makes the anastomosis line smaller, permitting completion within the safe 40 minutes time period. The left subclavian artery is re-attached using a small diameter graft at a later stage of the operation, either during the rewarming time or after termination of CPB. This technique not only permits quick and easy anastomoses, but also provides the surgeon with excellent suture-line access for inspection and additional hemostatic sutures if required later on (15-17).
For ascending and arch replacement, the mortality rate for elective cases was only 2%, and for all cases (including emergent cases) only 2.2%
In 1993 Svensson and associates reported their experience, which showed an increase in the risk of neurological impairment with prolonged DHCA times (18). This raised awareness about the adequacy of cerebral protection under deep hypothermia. However, in our experience, the overall stroke rate for patients undergoing ascending and arch operations with DHCA was 3.1% (15). Among the few patients with DHCA time longer than 45 minutes, the stroke rate was 13.1%. While it may be tempting to attribute this higher stroke rate in long arrest cases to inadequacy of cerebral protection, a closer look argues otherwise. Cases requiring such prolonged periods of circulatory arrest are by default more complex, with greater extent of the aneurysm disease, more severe calcification, and copious accumulation of debris in the aortic lumen and the arch branches. Consistent with this thesis, two-thirds of strokes in DCHA were embolic on CT scan (not ischemic), and thus not directly ascribable to the method of protection. Thus increased stroke rates in the prolonged group most probably represent the extent of the disease process itself, rather than reflecting directly on the adequacy of cerebral protection (15,16)
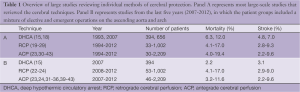
Review of published reports on other cerebral protection techniques (ACP, RCP) fails to identify superior survival and stroke rates with those alternate techniques (see Table 1). Panel A (Figure 1) summarizes the majority of reports in the literature on stroke and mortality rates for DHCA, ACP and RCP from studies that looked specifically at brain protection methods. It is interesting to note that among large-scale studies during the last five years (2007-2012) comparing patients that underwent ascending and arch surgery, our results with straight DHCA are very similar if not superior to other studies (Table 1, Panel B).
Full table
Several published reports have suggested that DHCA time of more than 20 minutes results in poor quality of life in patients undergoing thoracic aortic surgery in the postoperative period (44,45). However we did not share the same experience. We conducted a special study among patients with high cognitive demand in their professions undergoing aortic surgery with and without DHCA (The high cognitive demand patients included doctors, lawyers, scientists, administrators, teachers, musicians, artists, authors, and the like). This study specifically evaluated cognitive function before and after surgery. Cognitive function was assessed by a 21-part questionnaire adapted from A.F. Jorm’s Short Form IQCODE (Informant Questionnaire on Cognitive Decline in the Elderly) (46). We found no difference in preoperative and postoperative neuropsychometric responses from pre-DHCA to post-DHCA, and no difference between the groups operated with and without DHCA (Figure 4) (47). If any cognitive dysfunction was to occur, we surmise it would have manifested clearly among this group of patients, since cognition is vital to their occupation and daily duties. These results provide reassuring evidence that cognition does not suffer during hypothermic circulatory arrest.
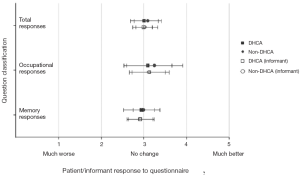
These findings are supported by other studies that looked at neurocognition after interventions on the ascending aorta and aortic arch with different cerebral protection techniques (48,49). In fact the Mount Sinai group (New York, NY) recently showed that DHCA (14-40 minutes) or isolated CPB (70-369 minutes) do not have an adverse effect on postoperative cognitive function. At the same time, selective cerebral perfusion (range, 39-83 minutes) was a significant predictor of decline in performance on memory and language tests (49).
In a particularly vivid example of the mental sharpness that we routinely see early post-operatively with straight DHCA, a 78 year-old woman, at 7 am the morning after her total aortic arch replacement, spells, without hesitation, the second author’s name (12 letters) - both forward and backward.
Comparison of DHCA with other techniques of cerebral protection
Strategies for cerebral protection during complex aortic surgery have evolved rapidly over the last two decades (1-3), making replacement of the aortic arch safe, almost routine procedures at many surgical centers. Experience is growing in relation to each of the three main protection techniques, straight DHCA, RCP and ACP. We have summarized the currently published and available data from large studies that have either reported their experience using one of the techniques or compared techniques at their institution (Table 2). We used mortality and stroke rates as the main outcome criteria of the published studies in order to permit comparisons. As can be seen in Table 2, the outcomes of using either DHCA, RCP or ACP are very similar, which supports the concept that all represent satisfactory options for cerebral protection. These studies permit no room for proselytizing that only one technique is acceptable. The small and inconsistent observed differences in reported mortality and stroke rates are expected and likely related to patient selection, disease complexity, and institutional variations. While we prefer straight DHCA at our own institution, as often the perfusion technique tends to become the operation itself, in contrast to the simplicity of DHCA, we feel that the other techniques are also very effective. We support their application based on institutional preferences.
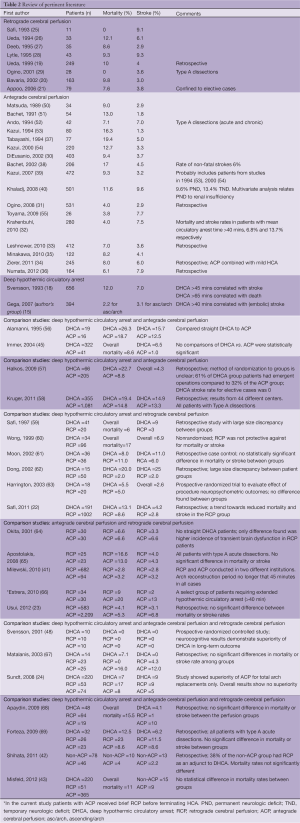
Full table
Of the three perfusion strategies, RCP is becoming increasingly less popular since there is published evidence that in reality very little oxygen actually reaches the brain tissue via venous perfusion (70,71). Thus the neuroprotective mechanism of RCP that has been shown in many studies with excellent results (19-23) may be due to achievement of cerebral hypothermia via venous brain cooling. As well, retrograde perfusion functions to flush embolic debris out of the arterial circulation system, which is a strong argument, not to be discounted, in favor of RCP. However, as our careful studies have shown, most patients with connective tissue disease of the ascending aorta are protected against arteriosclerosis, so debris is not frequently a problem. Also, we believe that the bloodless, wide-open exposure of the operative field enabled by straight DHCA permits the surgical team to avoid embolization in the first place, before it occurs, by visualizing and capturing debris during debridement and preparation of aortic cuffs for anastomosis (16).
Antegrade cerebral perfusion has gained enormous popularity in recent years. The concept of delivering oxygen-rich blood to the brain is extremely appealing as it is in a way the closest surrogate to simulate physiological brain perfusion. Many institutions have reported their experience and results with ACP, which has largely been excellent (23,24,30-34). However there are caveats and uncertainties about the actual technique of ACP. The number one debate is related to the question - “How many vessels should be perfused during ACP?” - since adequate and balanced perfusion of all brain structures is the cornerstone of effective cerebral protection. Some centers prefer to perfuse all three of the head vessels (35,36) including the left subclavian artery; some tend to perfuse just the right axillary and left common carotid artery (34,54); others even show evidence that unilateral perfusion (innominate only) is sufficient (33,57,72). The other extremely important aspect of ACP has to do with determining appropriate flow rates. Flowing at a high rate has been demonstrated to cause cerebral edema, while flowing at low rates will result in cerebral hypoperfusion, which by itself will compromise the entire purpose of the ACP technique. There is no universal agreement on the flow rates between institutions, thus the fine balance between hypo- and hyperperfusion is yet to be determined. Furthermore ACP has the potential to cause catheter-induced trauma to the head vessels, which are often fragile or dissected themselves. This is a major concern for cases of aortic dissection, which often extends into the vessels of the aortic arch. Lastly, there is always danger of air and particulate embolism during ACP; the latter may be incurred via dislodgement of atherosclerotic debris during catheter introduction. All these questions raise concerns about the risk-benefit balance of conducting ACP, especially during emergency cases of acute aortic dissection, when there may be little time to spare on establishing a sophisticated perfusion system of the brain.
With these issues in mind, straight DHCA does seem to us the most appealing method for protecting the brain, since it does not require introduction of any additional catheters, does not risk hyperperfusion with cerebral edema, incurs no trauma to the head vessels, and does not create a specific opportunity for embolism-related complications. Straight DHCA is especially appropriate in emergency situations such as life-threatening aortic dissections, when time is extremely precious. Our institutional experience of utilizing DHCA for the absolute majority of complex aortic cases has shown excellent results with mortality and stroke rates for ascending and arch cases as low as 2.2% and 3.1%, respectively. On the basis of our findings we strongly suggest that DHCA time of less than 40 minutes (and likely 60 minutes) can be considered safe with very little risk of postoperative neurologic complications (15-17,47).
Overall, based on the similarity of multi-institutional results presented in Table 2 it would probably be fair to state that multiple techniques and approaches for cerebral protection can be used with good results depending on the patient and the institution. No single technique has unequivocally demonstrated significant superiority over the others, with all offering advantages and liabilities (listed in Table 3).
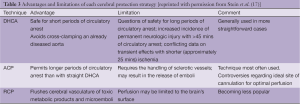
Full table
Conclusions
In conclusion, we strongly believe that straight DHCA is an effective and safe cerebral protection technique for the absolute majority of elective and emergency cases involving surgical interventions on the ascending aorta and the aortic arch. DHCA equals or surpasses other cerebral protection strategies in minimizing mortality and stroke rates and preserving postoperative cognitive function. Due to its convenience, simplicity and effectiveness, straight DHCA, we believe, can justifiably be a first-choice technique of cerebral protection. We prefer to focus on the operation itself rather than the perfusion techniques. The unencumbered surgical field of straight DHCA permits the surgeon to do the cutting and sewing without extraneous distractions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Elefteriades JA. What is the best method for brain protection in surgery of the aortic arch? Straight DHCA. Cardiol Clin 2010;28:381-7. [PubMed]
- Bachet J. What is the best method for brain protection in surgery of the aortic arch? Selective antegrade cerebral perfusion. Cardiol Clin 2010;28:389-401. [PubMed]
- Ueda Y. What is the best method for brain protection in surgery of the aortic arch? Retrograde cerebral perfusion. Cardiol Clin 2010;28:371-9. [PubMed]
- Lewis FJ, Taufic M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 1953;33:52-9. [PubMed]
- Karaskov AM, Litasova EE, Vlasov YA. A documentary on the life and work of Eugenij Nikolaevich Meshalkin. Circ Pathol Cardiac Surg 1999:4-11.
- Cooley DA, Mahaffey DE, De Bakey ME. Total excision of the aortic arch for aneurysm. Surg Gynecol Obstet 1955;101:667-72. [PubMed]
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Clarke DD, Sokoloff L. Circulation and Energy Metabolism of the Brain. In: Siegel GJ. editor. Basic neurochemistry molecular, cellular, and medical aspects. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 1999:637-70.
- Lassen NA. Autoregulation of Cerebral Blood Flow. Circ Res 1964;15:201-4. [PubMed]
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 2003;23:513-30. [PubMed]
- González-Ibarra FP, Varon J, López-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2011;2:4. [PubMed]
- Hägerdal M, Harp J, Nilsson L, et al. The effect of induced hypothermia upon oxygen consumption in the rat brain. J Neurochem 1975;24:311-6. [PubMed]
- Kirklin JW, Barratt-Boyes BG. eds. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications. 2nd ed. New York: Churchill Livingstone, 1993.
- Gega A, Rizzo JA, Johnson MH, et al. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84:759-66; discussion 766-7. [PubMed]
- Ziganshin B, Elefteriades JA. Does straight deep hypothermic circulatory arrest suffice for brain preservation in aortic surgery? Semin Thorac Cardiovasc Surg 2010;22:291-301. [PubMed]
- Stein LH, Elefteriades JA. Protecting the brain during aortic surgery: an enduring debate with unanswered questions. J Cardiothorac Vasc Anesth 2010;24:316-21. [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31. [PubMed]
- Ueda Y, Okita Y, Aomi S, et al. Retrograde cerebral perfusion for aortic arch surgery: analysis of risk factors. Ann Thorac Surg 1999;67:1879-82; discussion 1891-4.
- Bavaria JE, Brinster DR, Gorman RC, et al. Advances in the treatment of acute type A dissection: an integrated approach. Ann Thorac Surg 2002;74:S1848-52; discussion S1857-63.
- Appoo JJ, Augoustides JG, Pochettino A, et al. Perioperative outcome in adults undergoing elective deep hypothermic circulatory arrest with retrograde cerebral perfusion in proximal aortic arch repair: evaluation of protocol-based care. J Cardiothorac Vasc Anesth 2006;20:3-7. [PubMed]
- Safi HJ, Miller CC, Lee TY, et al. Repair of ascending and transverse aortic arch. J Thorac Cardiovasc Surg 2011;142:630-3. [PubMed]
- Usui A, Miyata H, Ueda Y, et al. Risk-adjusted and case-matched comparative study between antegrade and retrograde cerebral perfusion during aortic arch surgery: based on the Japan Adult Cardiovascular Surgery Database: the Japan Cardiovascular Surgery Database Organization. Gen Thorac Cardiovasc Surg 2012;60:132-9. [PubMed]
- Sundt TM 3rd, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg 2008;86:787-96; discussion 787-96. [PubMed]
- Safi HJ, Petrik PV, Miller CC 3rd. As originally published in 1993: Brain protection via cerebral retrograde perfusion during aortic arch aneurysm repair. Updated in 2001. Ann Thorac Surg 2001;71:1062-3; discussion 1064. [PubMed]
- Ueda Y, Miki S, Okita Y, et al. Protective effect of continuous retrograde cerebral perfusion on the brain during deep hypothermic systemic circulatory arrest. J Card Surg 1994;9:584-94; discussion 594-5. [PubMed]
- Deeb GM, Jenkins E, Bolling SF, et al. Retrograde cerebral perfusion during hypothermic circulatory arrest reduces neurologic morbidity. J Thorac Cardiovasc Surg 1995;109:259-68. [PubMed]
- Lytle BW, McCarthy PM, Meaney KM, et al. Systemic hypothermia and circulatory arrest combined with arterial perfusion of the superior vena cava. Effective intraoperative cerebral protection. J Thorac Cardiovasc Surg 1995;109:738-43. [PubMed]
- Ogino H, Ueda Y, Sugita T, et al. Surgery for acute type A aortic dissection using retrograde cerebral perfusion. Jpn J Thorac Cardiovasc Surg 2001;49:337-42. [PubMed]
- Di Eusanio M, Schepens MA, Morshuis WJ, et al. Antegrade selective cerebral perfusion during operations on the thoracic aorta: factors influencing survival and neurologic outcome in 413 patients. J Thorac Cardiovasc Surg 2002;124:1080-6. [PubMed]
- Ogino H, Sasaki H, Minatoya K, et al. Evolving arch surgery using integrated antegrade selective cerebral perfusion: impact of axillary artery perfusion. J Thorac Cardiovasc Surg 2008;136:641-8; discussion 948-9. [PubMed]
- Krähenbühl ES, Clément M, Reineke D, et al. Antegrade cerebral protection in thoracic aortic surgery: lessons from the past decade. Eur J Cardiothorac Surg 2010;38:46-51. [PubMed]
- Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90:547-54. [PubMed]
- Zierer A, Detho F, Dzemali O, et al. Antegrade cerebral perfusion with mild hypothermia for aortic arch replacement: single-center experience in 245 consecutive patients. Ann Thorac Surg 2011;91:1868-73. [PubMed]
- Minakawa M, Fukuda I, Yamauchi S, et al. Early and long-term outcome of total arch replacement using selective cerebral perfusion. Ann Thorac Surg 2010;90:72-7. [PubMed]
- Numata S, Tsutsumi Y, Monta O, et al. Aortic arch repair with antegrade selective cerebral perfusion using mild to moderate hypothermia of more than 28 ℃. Ann Thorac Surg 2012;94:90-5; discussion 95-6. [PubMed]
- Tabayashi K, Ohmi M, Togo T, et al. Aortic arch aneurysm repair using selective cerebral perfusion. Ann Thorac Surg 1994;57:1305-10. [PubMed]
- Bachet J, Guilmet D. Brain protection during surgery of the aortic arch. J Card Surg 2002;17:115-24. [PubMed]
- Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg 2007;83:S796-8; discussion S824-31.
- Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14. [PubMed]
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57. [PubMed]
- Shihata M, Mittal R, Senthilselvan A, et al. Selective antegrade cerebral perfusion during aortic arch surgery confers survival and neuroprotective advantages. J Thorac Cardiovasc Surg 2011;141:948-52. [PubMed]
- Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8. [PubMed]
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90; discussion 1891-4.
- Immer FF, Lippeck C, Barmettler H, et al. Improvement of quality of life after surgery on the thoracic aorta: effect of antegrade cerebral perfusion and short duration of deep hypothermic circulatory arrest. Circulation 2004;110:II250-5. [PubMed]
- Jorm AF. IQCODE: Informant Interviews. In: Copeland JR, Abou-Saleh MT, Blazer DG. eds. Principles and practice of geriatric psychiatry. 2nd ed. Chichester, West Sussex, UK; New York, NY, USA: John Wiley & Sons, 2002: 141-2.
- Percy A, Widman S, Rizzo JA, et al. Deep hypothermic circulatory arrest in patients with high cognitive needs: full preservation of cognitive abilities. Ann Thorac Surg 2009;87:117-23. [PubMed]
- Svensson LG, Nadolny EM, Penney DL, et al. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Ann Thorac Surg 2001;71:1905-12. [PubMed]
- Uysal S, Lin HM, Fischer GW, et al. Selective cerebral perfusion for thoracic aortic surgery: association with neurocognitive outcome. J Thorac Cardiovasc Surg 2012;143:1205-12. [PubMed]
- Matsuda H, Nakano S, Shirakura R, et al. Surgery for aortic arch aneurysm with selective cerebral perfusion and hypothermic cardiopulmonary bypass. Circulation 1989;80:I243-8. [PubMed]
- Bachet J, Guilmet D, Goudot B, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102:85-93; discussion 93-4. [PubMed]
- Ando M, Nakajima N, Adachi S, et al. Simultaneous graft replacement of the ascending aorta and total aortic arch for type A aortic dissection. Ann Thorac Surg 1994;57:669-76. [PubMed]
- Kazui T, Kimura N, Yamada O, et al. Surgical outcome of aortic arch aneurysms using selective cerebral perfusion. Ann Thorac Surg 1994;57:904-11. [PubMed]
- Kazui T, Washiyama N, Muhammad BA, et al. Total arch replacement using aortic arch branched grafts with the aid of antegrade selective cerebral perfusion. Ann Thorac Surg 2000;70:3-8; discussion 8-9. [PubMed]
- Toyama M, Matsumura Y, Tamenishi A, et al. Safety of mild hypothermic circulatory arrest with selective cerebral perfusion. Asian Cardiovasc Thorac Ann 2009;17:500-4. [PubMed]
- Alamanni F, Agrifoglio M, Pompilio G, et al. Aortic arch surgery: pros and cons of selective cerebral perfusion. A multivariable analysis for cerebral injury during hypothermic circulatory arrest. J Cardiovasc Surg (Torino) 1995;36:31-7. [PubMed]
- Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg 2009;138:1081-9. [PubMed]
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [PubMed]
- Safi HJ, Letsou GV, Iliopoulos DC, et al. Impact of retrograde cerebral perfusion on ascending aortic and arch aneurysm repair. Ann Thorac Surg 1997;63:1601-7. [PubMed]
- Wong CH, Bonser RS. Retrograde cerebral perfusion: clinical and experimental aspects. Perfusion 1999;14:247-56. [PubMed]
- Moon MR, Sundt TM 3rd. Influence of retrograde cerebral perfusion during aortic arch procedures. Ann Thorac Surg 2002;74:426-31; discussion 431. [PubMed]
- Dong P, Guan Y, He M, et al. Clinical application of retrograde cerebral perfusion for brain protection during surgery of ascending aortic aneurysm--a report of 50 cases. J Extra Corpor Technol 2002;34:101-6. [PubMed]
- Harrington DK, Bonser M, Moss A, et al. Neuropsychometric outcome following aortic arch surgery: a prospective randomized trial of retrograde cerebral perfusion. J Thorac Cardiovasc Surg 2003;126:638-44. [PubMed]
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9. [PubMed]
- Apostolakis E, Koletsis EN, Dedeilias P, et al. Antegrade versus retrograde cerebral perfusion in relation to postoperative complications following aortic arch surgery for acute aortic dissection type A. J Card Surg 2008;23:480-7. [PubMed]
- Estrera AL, Miller CC, Lee TY, et al. Integrated cerebral perfusion for hypothermic circulatory arrest during transverse aortic arch repairs. Eur J Cardiothorac Surg 2010;38:293-8. [PubMed]
- Matalanis G, Hata M, Buxton BF. A retrospective comparative study of deep hypothermic circulatory arrest, retrograde, and antegrade cerebral perfusion in aortic arch surgery. Ann Thorac Cardiovasc Surg 2003;9:174-9. [PubMed]
- Apaydin AZ, Islamoglu F, Askar FZ, et al. Immediate clinical outcome after prolonged periods of brain protection: retrospective comparison of hypothermic circulatory arrest, retrograde, and antegrade perfusion. J Card Surg 2009;24:486-9. [PubMed]
- Forteza A, Martín C, Centeno J, et al. Acute type A aortic dissection: 18 years of experience in one center (Hospital 12 de Octubre). Interact Cardiovasc Thorac Surg 2009;9:426-30. [PubMed]
- Ergin MA, Griepp EB, Lansman SL, et al. Hypothermic circulatory arrest and other methods of cerebral protection during operations on the thoracic aorta. J Card Surg 1994;9:525-37. [PubMed]
- Reich DL, Uysal S, Ergin MA, et al. Retrograde cerebral perfusion as a method of neuroprotection during thoracic aortic surgery. Ann Thorac Surg 2001;72:1774-82. [PubMed]
- Urbanski PP, Lenos A, Zacher M, et al. Unilateral cerebral perfusion: right versus left. Eur J Cardiothorac Surg 2010;37:1332-6. [PubMed]





