Treatment of lone atrial fibrillation: minimally invasive pulmonary vein isolation, partial cardiac denervation and excision of the left atrial appendage
Approximately 14 years ago, the treatment strategies for atrial fibrillation (AF) began to change and diverge. Some electrophysiologists initiated catheter ablation for lone (structurally normal heart) AF (1). Reported success with the Haïssaguerre technique led some cardiac surgeons to begin concomitant surgical left atrial isolation for treatment of AF as an adjunct to open heart surgery for valve or coronary artery bypass graft procedures.
Since 1999, my colleagues and I have diligently pursued a minimally invasive surgical, beating-heart, left atrial isolation technique that is offered to patients with lone AF. We began clinical cases in 2003. In 2005, we reported our initial experience with video-assisted bilateral pulmonary vein (PV) isolation and left atrial appendage (LAA) exclusion for the minimally invasive treatment of AF (Wolf technique) (2). PV isolation was achieved bilaterally using a dry radio frequency device (Atricure, Cincinnati, Ohio, USA). The LAA was removed with a surgical stapler (Ethicon Endosurgery, Cincinnati, Ohio, USA). At average 3-month follow-up in 23 patients, 21 were free of AF by objective endpoints [Electrocardiograph (ECG) and 7-day home monitoring]. The short-term cure rate was 91%.
In 2004, we then began two projects simultaneously: first, we sought to demonstrate this minimally invasive surgical technique to other thoracic surgeons, and second, we investigated options for new instruments that could improve the technique. During the last eight years we have added pacing for block, testing entrance and exit block, ganglionic plexi testing and isolation (3), and use of a bipolar pen for isolation.
After demonstrating the technique to surgeons in the United States, Europe, South America, and Asia, there emerged more single-center reports using our technique, with similar good short-term results (4-6). This type of minimally invasive surgical technique to cure AF is becoming more common. More than 12,000 minimally invasive Wolf techniques with the Atricure system have been performed to date.
Recently, the Atrial Fibrillation Catheter Ablation Versus Surgical Ablation Treatment (FAST) Trial—the first randomized, controlled study comparing minimally invasive surgical ablation consisting of bilateral left atrial antral isolation, partial cardiac denervation and excision of the LAA to catheter ablation—was reported with 1-year follow-up (7). Even though 67% of patients had a previous catheter ablation, the surgical results were far superior to catheter ablation with respect to absence of AF at one year (65.6% for the surgical procedure vs. 36.5% for catheter ablation). In addition, all surgical patients had removal of the LAA, obviating the need for continued anticoagulation (8).
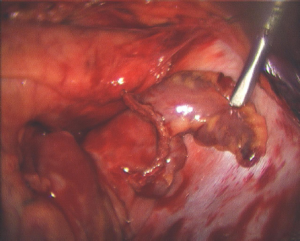
It should be clearly understood that energy sources that seem to perform adequately during on-pump procedures with the heart empty may not provide transmural lesions in the beating heart (9). However, dry bipolar RF produces in complete transmural lesions and no histological damage to surrounding tissues on the beating heart (Figure 1).
These findings are explained by the fact that circulating blood in the beating heart acts as an infinite heat sink, making transmural lesions problematic. Using a bipolar clamp and dry RF, there is no heat sink as the blood is excluded from the treated area. In addition, in using a dry bipolar clamp there is minimal lateral thermal spread, so the energy application is limited to the treated area.
Addressing the left atrial appendage
The various “minimally invasive” techniques currently being used also differ in LAA treatment. We have been a strong proponent of LAA exclusion or excision.
Some patients are referred because of inability to tolerate warfarin on the background of transient ischemic attacks (TIAs) or stroke. The main reason for surgical referral in this subgroup of patients is to exclude the LAA as a source of repeated cerebral embolic events. In addition, until we achieve 100% sinus rhythm as defined by the published guidelines [such as 2012 HRS/EHRA/ECAS (10)] with these minimally invasive surgical AF techniques, it seems prudent to reduce the stroke rate by excluding or removing the LAA. In the FAST trial, there were two cerebral vascular events in the catheter ablation group in the postoperative 12-month period. These problems are absent in the postoperative minimally invasive surgical patients, as the LAA has been removed (8).
Intraoperative electrophysiologic testing
We believe intraoperative EP testing is of paramount importance. We were the first to test for ganglionated plexi (GP) activity about the left atrium and isolate these GPs during our minimally invasive AF procedure, with the assistance of Dr. Ben Scherlag, and using his techniques (11-13). A map has been developed to record the epicardial position of these GPs in each patient (Figure 2A,B).
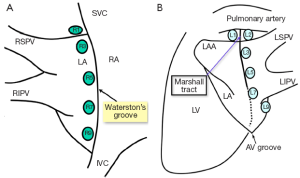
The GPs are isolated both with the bipolar clamp applied medial to Waterston groove fat pad and with a bipolar pen directly over any remaining positive GPs. We also test after isolation of the GPs. This second part of the intraoperative EP testing is designed to objectively document PV block after PV isolation. In addition to adding objectivity to the procedure, by learning these EP techniques, the surgeon will speak the same language as the electrophysiologist.
In cases referred after failed catheter ablation for AF, the nonisolated PVs are identified at the time of testing during the minimally invasive surgical AF procedure. In addition to providing a map for the surgical ablation procedure, this provides helpful feedback to the electrophysiologist and helps ensure consistency when reporting results. The GP isolation also addresses one of the plausible mechanisms of AF.
Patient selection and preoperative testing
The ideal patient for minimally invasive surgical treatment is listed below:
- History of TIA or stroke;
- Inability to take anticoagulants;
- Patient desire to be rid of anticoagulants or antiarrhythmics;
- Documented AF;
- No significant valvular disease;
- No significant obstructive coronary artery disease;
- Left atrium less than 6 cm;
- Failed catheter ablation.
AF must be objectively documented preoperatively. If it has not been, 7-day continuous Holter monitoring is recommended. We have found other arrhythmias in patients labeled with AF, including recurrent non-sustained ventricular tachycardia. These patients must be referred for proper work-up and certainly would not benefit from a surgical AF procedure. Any history of obstructive CAD or valvular heart disease must be thoroughly evaluated preoperatively.
Our routine preoperative workup is described in Table 1.

Full table
Surgical approach
Although catheter-based techniques are the least invasive approach to the heart, it can be argued that epicardial discrete surgical isolation of the antrum (as opposed to current extensive endocardial ablative techniques) is much less invasive to the heart itself. This difference in approach helps to account for the low pro-arrhythmia rate following minimally invasive antrum exclusion surgically with a clamp versus catheter ablation (personal observation). Another advantage of the surgical approach is that it reliably treats the autonomic nerves, which are epicardial (3,13).
Preparation
A double lumen endotracheal tube is used for selective lung ventilation and a central line is placed. External defibrillator pads are placed in the appropriate vector. Sequential compression stockings are placed on the lower extremities. A warmer is used to control body temperature during the procedure.
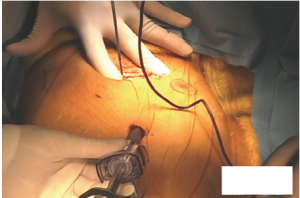
Right side: place the patient in the left lateral decubitus position at 45-60°, with the right arm on an arm holder (Allen Medical Systems, Acton, MA, USA). Document the external anatomy. Place the first port in the sixth or seventh intercostal space (ICS) in the mid-axillary line. From the scope view, identify the fourth ICS. Make a 4-6 cm working port in the auscultatory triangle in the fourth ICS and carry it anteriorly (Figure 3).
Initial dissection
Bluntly develop a space just inferior to the IPV and lateral to the IVC into the oblique sinus. Verify that a pediatric Yankauer suction freely falls into the oblique sinus through this opening. Use the scope to check the access angle for the dissector device. A sponge stick can be used to gently retract the heart medially as needed for access and visualization. Roll the SVC medially with the suction or with a grasper, but do not grasp the SVC.
The Lumitip™ Dissector
Create a second working port (10 mm) either medially or laterally to the scope port for dissector use. This allows the dissector to be lined up for direct in-line access with the appropriate port.
Dissector placement: Bluntly retract the SVC medially with a sponge stick or grasper to help gain exposure and visualization of the superior aspect of the RPA. Feed the dissector tip into the oblique sinus just above the IVC, around the back of the heart, and then articulate the dissector to pass between the superior PV and the pulmonary artery. Advance the Isolator™ lower jaw until its posterior tip is visible superior to the RSPV, making sure to apply adequate tension to the red rubber catheter to help lead the lower jaw into place.
Ablation
Right side: transmural and contiguous lesions are created to electrically isolate the antrum from the remainder of the heart. On the right side during clamp applications, perform additional three burns after herniating the Waterson’s groove fat pad into the clamp. This provides for more medial isolation of the left antrum and usually treats all the GPs, except level 9 on the right (Figure 4).
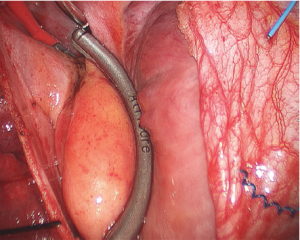
Map and record the epicardial ECG. If the ECG recording shows quiescence in all locations on the PVs, then PV antrum isolation is complete. Prior to closing the pericardium, a temporary bipolar pacing lead is placed through the medial edge of the pericardium to lie on the right atrium. The leads are held in place by the pressure of the pericardium on the right atrium. Place a small drain through one of the inferior port sites and close the right-sided incisions.
Left side: the same patient position is utilized and the access ports and exposure are identical to the right side. The dissection and ablation are performed similar to the right side, except that on the left side, the ligament of Marshall must be divided. Passing and placement of the Isolator clamp is similar to the right side (Figure 5).
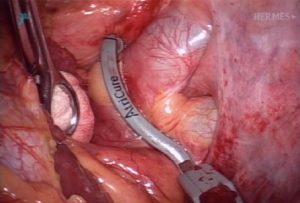
Exclusion of the left atrial appendage (LAA)
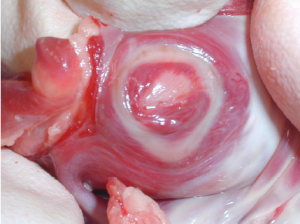
After the left PVs have been isolated, the LAA is excluded. The Echelon flex 60 powered stapler (Ethicon Endosurgery) is used with a thick tissue load (green, staple height 4.8 mm). The stapler should be introduced through the most posteriorly located inferior port site. The stapler is fired across the base of the LAA to excise the entire LAA. The pericardium should be closed on the left side to avoid herniation of the heart (Figure 6).
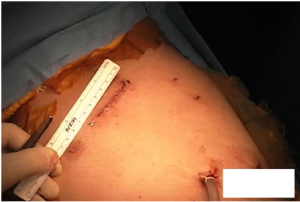
At the conclusion of the left side there are two inferior port sites, one of which is used for the drain tube. The working port incision in the 4th ICS measures about 6 cm (Figure 7).
Conclusions
Recently we reviewed 157 patients who are now 1 to 9 years out from the Wolf procedure. The patients’ ages ranged from 15 to 87 years old. The cure rate for paroxysmal AF was 92%, for persistent AF, 85% and for long-standing persistent AF, 75%. The follow-up included 7-day continuous monitoring. There were no deaths (personal review).
Caveats
Preoperative 64 slice CT scan with 60 seconds delayed imaging is superior to TEE for detecting LAA thrombus (14).
If it is difficult to discern thrombus in the distal LAA, start the minimally invasive surgical procedure on the left side and remove the LAA first. Although we occasionally do this to be conservative when there is some question of LAA opacification, we have yet to find thrombus in the LAA. If there is questionable opacification distal to the left circumflex, in our practice it has been safe to perform the procedure.
Remove the LAA flush with the left atrium in all cases. Remember there are two reasons for LAA excision: (I) to dramatically decrease the risk of AF stroke and (II) to eliminate AF drivers in the LAA.
Test and ablate the GPs in all cases. This treats a second plausible source of AF, the so-called vagal AF.
On the right side during clamp applications, perform three additional burns after herniating the Waterson’s groove fat pad into the clamp. This provides for more medial isolation of the left antrum and usually treats all the GPs, except level 9 on the right.
Place a bipolar pacing lead inside the pericardium on the right side. Before the minimally invasive surgical procedure, some patients have been continuously out of rhythm for more than a year. These patients are often on medications that poison the conduction system in order to slow the ventricular response to AF. When patients in such a state are cardioverted, they often have slow conduction. Temporary pacing for 1-5 days allows time for the conduction system to recover while medications are held.
Place external defibrillation pads on the patient before prepping; one pad over the upper middle back and one pad on the anterior midline just below the xiphoid. Keep the defibrillation machine on synch mode.
Arrange proper follow-up, including antiarrhythmic medications if needed and a post-op cardioversion if indicated. Follow-up for at least one year is recommended, especially for patients with continuous AF preoperatively. These patients must understand that resuming sinus rhythm is a process that starts with the minimally invasive surgical procedure but may include postoperative antiarrhythmic treatment for 3-12 months and post-operative cardioversion. We inform our patients that the surgical procedure is the beginning of a process to restore sinus rhythm.
Ensure antiarrhythmic medications are weaned postoperatively at an appropriate time.
If after one year postoperatively the patient is out of rhythm after antiarrhythmics and cardioversion have failed, consider catheter ablation. In our experience, catheter ablation postoperatively can render the patient AF free.
Treat postoperative typical atrial flutter aggressively with right-sided catheter ablation. It works.
Previous CABG is not a contraindication to the Wolf technique, provided there is no graft in the vicinity of the LAA. A patent circumflex graft is a contraindication to the minimally invasive surgical procedure.
Some patients benefit from simple LAA excision. These are patients that are not good candidates for the Wolf technique, but cannot take anticoagulants.
Patients who have been continuously out of rhythm for even seven years are able to maintain sinus rhythm after the minimally invasive surgical procedure if the LA is less than 5.5 cm preoperatively.
Patients with a left atrium measuring greater than 5 cm are able to maintain sinus rhythm after the minimally invasive surgical procedure if the patient has had some sinus rhythm within the last year or so.
The treatment to eliminate AF continues to evolve rapidly and patients are benefitting from the tremendous amount of focus on this common problem.
Acknowledgements
Disclosure: The author receives a royalty and proctoring fees from Atricure Inc. The author declares no conflict of interest.
References
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [PubMed]
- Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797-802. [PubMed]
- Mehall JR, Kohut RM Jr, Schneeberger EW, et al. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg 2007;83:538-41. [PubMed]
- Wudel JH, Chaudhuri P, Hiller JJ. Video-assisted epicardial ablation and left atrial appendage exclusion for atrial fibrillation: extended follow-up. Ann Thorac Surg 2008;85:34-8. [PubMed]
- Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol 2007;20:89-93. [PubMed]
- McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:1289-95. [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [PubMed]
- Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. [PubMed]
- Aupperle H, Doll N, Walther T, et al. Histological findings induced by different energy sources in experimental atrial ablation in sheep. Interact Cardiovasc Thorac Surg 2005;4:450-5. [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [PubMed]
- Patterson E, Po SS, Scherlag BJ, et al. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624-31. [PubMed]
- Scherlag BJ, Yamanashi W, Patel U, et al. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol 2005;45:1878-86. [PubMed]
- Schauerte P, Scherlag BJ, Patterson E, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592-9. [PubMed]
- Meyer CA, Hall JE, Mehall JR, et al. Impact of Preoperative 64-Slice CT Scanning on Mini-Maze Atrial Fibrillation Surgery. Innovations (Phila) 2007;2:169-75. [PubMed]