Surgical atlas of thoracoscopic lobectomy and segmentectomy
Video-assisted thoracoscopic surgery (VATS) is a well-established technique for major pulmonary resections (1). Since the first procedure was performed more than 20 years ago, the operative approach and instrumentation have matured. In 2007, CALGB 39802 trial established the most authoritative and accepted definition of the VATS lobectomy technique, i.e., 4-8 cm access incision, totally endoscopic approach, without rib spreading and individual anatomical dissection and division of pulmonary vein, artery and bronchus (2). Compared to open surgery, the minimally invasive approach has a number of benefits especially in the immediate post-operative period (3). A recent meta-analysis of propensity score matched patients demonstrated significantly lower incidences of overall complications, prolonged air leak, pneumonia, atrial arrhythmias and renal failure, as well as shorter hospitalization compared to open thoracotomy (4). This study further consolidated the benefits of VATS and offered the highest clinical evidence on this topic.
The posterior approach was first developed by Mr. William Walker from Edinburgh in April 1992. In contrast to the anterior approach, the main differences in techniques of the posterior approach include: (I) the surgeons stand posterior to the patient; (II) the utility incision is made at the 6th or 7th intercostal space anterior to latissimus dorsi muscle, instead of the 4th intercostal space; (III) the camera port is made through the auscultatory triangle, instead of lower anterior incision; and (IV) the order of dissection is from posterior to anterior, by opening up the fissure first to identify and isolate pulmonary arterial branches. The main advantages of the posterior approach include: (I) easy access to posterior hilum; (II) lymph nodes are clearly visualized; and (III) tips of the instruments are coming towards the camera, which allows safer dissection. The fact that the posterior hilum can be clearly seen greatly facilitates dissection of the segmental bronchial branches and pulmonary arteries. Hence, the posterior approach offers great advantages for VATS segmentectomy.
Preoperative considerations
I have adopted VATS resection as the preferred surgical strategy of choice for all cases of peripheral lung carcinoma of 7 cm or less in diameter and for suitable benign disease. Lobectomy and anatomic segmentectomy are standard procedures. It is possible to utilize VATS techniques in patients with more advanced disease such as moderate chest wall or pericardial involvement and, rarely, for pneumonectomy in patients with low bulk hilar involvement. However, with the trend towards lung conservation strategies, we now reserve pneumonectomy for individuals in whom bronchovascular reconstruction is not feasible.
Baseline pulmonary function is assessed by using a combination of spirometry and CO transfer factors. Additionally, selected patients undergo exercise testing. Cardiological assessment is carried out as relevant to the individual patient. Echocardiography assessment of pulmonary (PA) pressure is undertaken in patients at risk of pulmonary hypertension (PAP >45 mmHg). Few patients are declined surgery on the basis of poor pulmonary function data (e.g., both FEV1 and FVC <35%) (1). In addition to a contrast-enhanced computed tomography scan of the head, chest, abdomen and pelvis, positron emission tomography-CT (PET-CT) with 18F-fluordeoxyglucose (18F-FDG) is performed in all patients with bronchogenic carcinoma under consideration for resection. In patients considered suitable for lobectomy or segmentectomy, the VATS approach is attempted in all patients meeting size and stage criteria. The only absolute contraindications are those patients in whom the pleural cavity is obliterated on radiological grounds or who clearly have very proximal disease requiring a pneumonectomy. The requirement for sleeve lobectomy is a significant relative contraindication, but not absolute.
Operative techniques
Anesthesia and positioning
Following induction of anesthesia, the patient is positioned in the lateral decubitus position. The hands are placed unsupported in the “prayer” position in front of the face and the operating table is manipulated to extend the thorax laterally opening up the intercostal spaces. As soon as the double lumen endotracheal tube is confirmed to be in the correct position, whilst the patient is still in the anaesthetic room, ventilation is switched to the contralateral lung to optimize deflation of the lung that is to be operated upon. Suction is occasionally used if the lung does not deflate readily. The respiratory rate can be increased to 20 breaths/min or more in order to reduce the tidal volume and hence the degree of mediastinal excursion due to ventilation. This provides a more stable operating field. Central lines or urinary catheters are rarely used, but always use an arterial line and large bore venous cannulae.
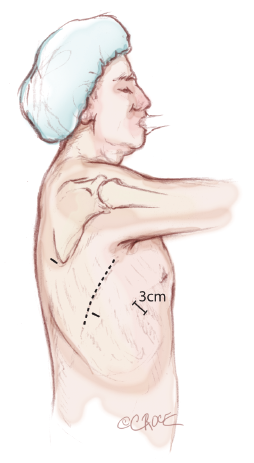
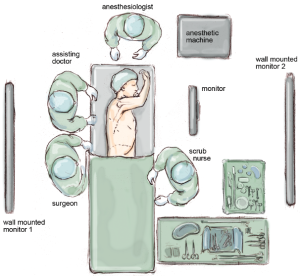
The paravertebral catheter is inserted as soon as the chest cavity is entered, under thoracoscopic guidance. This is used for perioperative analgesia in preference to epidural anaesthesia and it remains in place for 48 hours. Furthermore, a patient-controlled pump is supplied to the patient for post-operative analgesia. The positioning of the surgical, anaesthetic and nursing teams and the equipment is illustrated in Figure 1. The surgeon and their assistants stand at the patient’s back with the screen directly across the table and the scrub nurse obliquely opposite.
Instrument
I prefer a zero degree 5 mm high definition STORZ video thoracoscope, as it provides a single axis view allowing easy correction of orientation. A combination of endoscopic and standard open surgical instruments is used. Lung retraction and manipulation are performed using ring-type sponge-holding forceps. Long artery dissection forceps (30 cm) with or without mounted pledgets are employed for blunt dissection, which are particularly useful for exposing the PA at the base of the oblique fissure, cleaning structures and clearing node groups. A range of curved forceps and an endodissector are used gently as probes to create a passage between the lung parenchyma and major hilar structures. A right-angled dissector or long curved artery forceps is used to dissect out and pass slings around pulmonary arteries and veins. Endoscopic clips are used to ligate small vessels whilst large vessels and lung parenchyma are divided using endoscopic stapling devices to ensure haemostasis and aerostasis. Both endoscopic shears and specific VATS Metzenbaum type scissors to be helpful. The latter have the advantage of curved blade ends, which reduce the risk of vascular injury.
Incision
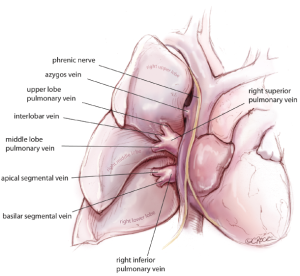
Three access ports are used and port position is standard irrespective of the lobe or segment to be removed (Figure 2). A 3-4 cm utility port site incision is made in the sixth or seventh intercostal space (whichever is the wider). The camera is temporarily introduced through this port to facilitate safe creation of a 0.5 cm incision posteriorly in the auscultatory triangle at the point nearest to the upper end of the oblique fissure. The anterior hilum dissection is not essential for the posterior approach. However, for completeness of this article, it is important to understand the segmental anatomy of the pulmonary veins viewed from the anterior hilum. The pulmonary veins are the most anterior structures in the hilum (Figure 3). Their tributaries are also anterior to the segmental arteries and bronchi. The interlobar vein often traverses between the upper and lower lobes in the oblique and then the upper and middle lobes in the horizontal fissure before joining the superior pulmonary vein in the hilum. In majority of cases, the middle lobe vein drains into the right superior pulmonary vein.
A port is inserted to accommodate the camera, which is positioned in the auscultatory triagle for the remainder of the procedure. A further 1 cm port is created in the mid-axillary line level with the upper third of the anterior utility port. The anterior and posterior ports lie at opposite ends of the oblique fissure. A video-imaged thoracoscopic assessment is performed to confirm the location of the lesion, establish resectability and exclude unanticipated disease findings that might preclude resection. If the lesion is small or cannot be palpated easily, sound knowledge of segmental anatomy is crucial for determining the location of the lesion within the segment(s) of the respective lobe.
The ‘landmark’ lymph node
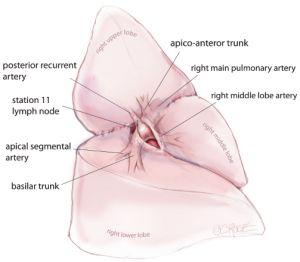
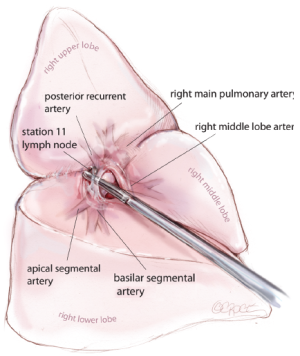
The first step is to identify the PA within the central section of the oblique fissure. In some patients the PA is immediately visible, but in the majority of cases, the PA is revealed by separating the overlying pleura using blunt dissection with mounted pledgets. If the fissure does not open easily or is fused, an alternative approach utilizing a fissure-last dissection should be considered. Once the PA has been identified, the sheath of the artery is grasped with a fine vascular clamp or long artery forceps and an endoscopic dissector is used to enter the sheath defining the anterior and posterior margins of the artery. The apical lower branch of the PA is often exposed during this dissection (Figure 4).
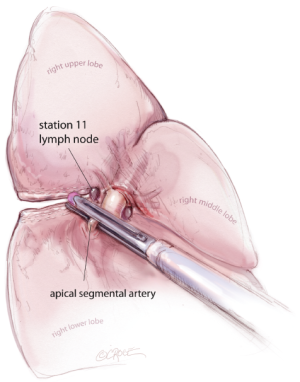
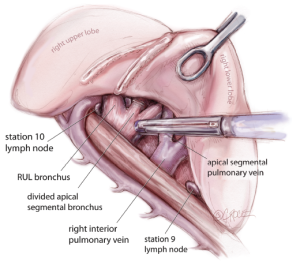
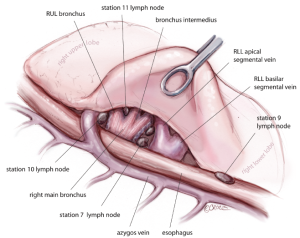
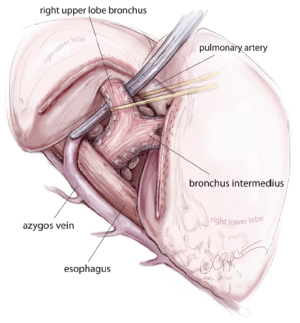
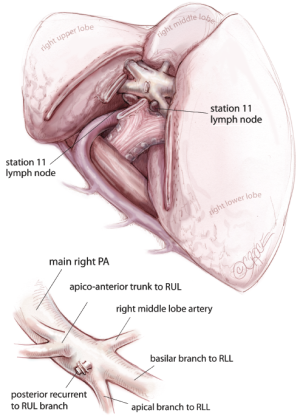
For all lobectomy and segmentectomy procedures excepting middle lobectomy, the lung is then reflected anteriorly and the posterior pleural reflection is divided using sharp and blunt dissection. On the right, this process should clear lung tissue away from the angle between the bronchus intermedius and the upper lobe bronchus, exposing the posterior hilar lymph nodes in this position (Figure 5). One lymph node packet, the station 11 lymph node, sitting at the bronchial bifurcation between the right upper lobe and the bronchus intermedius is the ‘landmark’ lymph node to me, because just superficial to this, it indicates a safe passage from the interlobar fissure to the posterior hilum over the pulmonary artery. From the anterior port site, dissecting forceps are passed gently immediately superficial and posterior to this station 11 ‘landmark’ lymph node, where it has been identified in the oblique fissure (Figure 6). When the lung is retracted anteriorly, the tips of the long artery forceps will emerge through the incised posterior pleural reflection, above the ‘landmark’ lymph node that is now viewed from the posterior hilum. This maneuver is the key step for any VATS lobectomy or segmentectomy via the posterior approach on the right side. Care should be taken during this maneuver not to disrupt this lymph node lying on the bronchial bifurcation. A sling is then passed behind the posterior fissure, which is divided with an endoscopic linear stapling device. The PA is now clearly seen and the distinction between the upper and lower lobes is established. Dissection then proceeds according to the lobe or segment to be resected.
Right upper lobectomy
Having divided the posterior fissure, the posterior ascending segmental branch of the PA is often evident, and should be divided at this stage if appropriate. It is frequently small enough to clip. The upper lobe bronchus is then identified and dissected out. It is common to find a substantial bronchial artery running alongside the bronchus, which should be ligated with clips and divided. Note that clips are only used on the proximal end and the distal end is not clipped since clips in this position may interfere with subsequent stapling of the bronchus. The upper lobe is then retracted inferiorly and blunt dissection with mounted pledgets is used to free the cranial border of the upper lobe bronchus and define the apico-anterior trunk. The azygos vein is often closely related to the bronchus and can be pushed away using a gentle sweeping motion. Long artery forceps are passed around the upper lobe bronchus close to its origin in the plane between the bronchus and the associated node packet (Figure 7). It should be appreciated that the apico-anterior trunk lies immediately anterior to the bronchus, but sometimes separated by station 11 right upper lobe lymph nodes. The bronchus is transected at this level using an endoscopic linear stapling device. It is not necessary to inflate the lung to test that the correct bronchus is being divided, as the vision is invariably excellent via the posterior approach and the re-inflated lung may subsequently obscure the view for remainder of the resection.
Following division of the bronchus, the feeding vessels to the right upper lobe bronchus node packet are clipped and divided, allowing the nodes to be swept up into the operative specimen. Clasping the distal end of the transected bronchus with an endoscopic toothed grasper, the upper lobe can be reflected upwards. The posterior segmental artery is divided at this stage if not already dealt with and the apical and anterior segmental arteries or common stem artery are carefully cleaned, dissected out (Figure 8) and divided with an endoscopic stapler. Finally, the lung is retracted posteriorly facilitating dissection of the superior vein. This can be divided from either the posterior or anterior aspect as convenient, taking care in either case to identify clearly and preserve the middle lobe vein. The transverse fissure is then divided. The middle lobe artery is most easily identified and protected if the stapling device is first passed through the inferior port and fired from posterior to anterior. Division of the transverse fissure is then completed, passing the stapling device through the anterior port. The inferior pulmonary ligament is divided to facilitate expansion of the right lower lobe.
Right lower lobectomy
Having identified the PA in the oblique fissure and divided the posterior oblique fissure, the pulmonary artery is then divided either in one or separately as a basal trunk artery and the apical segmental artery to the lower lobe. The space between the superior and inferior veins is developed and a long clamp is passed into this space emerging anterior to the PA in the oblique fissure. A sling is passed into this plane and the anterior oblique fissure is then divided. The lower lobe is mobilized by dividing the inferior pulmonary ligament. The inferior vein is dissected free from surrounding tissue and divided using an endoscopic linear stapling device. The bronchus is identified and the bronchial vessels are clipped proximally. Lymph nodes are cleared from its medial and lateral margins. The lower lobe bronchus is divided through its apical and basal branches preserving airflow to the middle lobe. The middle lobe bronchus must be visualized prior to stapling.
Right middle lobectomy
The PA is identified and the anterior oblique fissure is divided as for right lower lobectomy. The vein, bronchus and arteries are then seen clearly, like three little ‘soldiers’ when the right upper lobe is retracted superiorly and are divided in sequence. The transverse fissure is divided as described for right upper lobectomy.
Left upper lobectomy
The PA is identified in the oblique fissure and the posterior aspect of the oblique fissure is divided in a similar way to the right side. The arterial branches to the left upper lobe are then divided sequentially. Division of the anterior aspect of the fissure is completed in similar manner to that on the right side. It is important to develop the space between the pulmonary veins and central to the fused anterior oblique fissure thoroughly. When passing a clamp through the utility incision and under the fused fissure, the surgeon will feel the lower lobe bronchus and should allow the clamp to pass superficially in order to preserve the airway to the lower lobe. Gentle blunt dissection is used to separate the superior pulmonary vein from the anterior surface of the bronchus. A long clamp is passed around the base of the bronchus, taking particular care not to damage the PA. Retraction of the PA using a mounted pledget may be helpful. A sling is passed around the bronchus and used to elevate it (crane maneuver) in relation to the pulmonary artery and create a space via which an endoscopic stapling device can be inserted to divide the bronchus. The superior vein is cleaned and divided. The inferior pulmonary ligament is divided up to the level of the inferior vein to facilitate expansion of the lower lobe.
Left lower lobectomy
As on the right side, having identified the PA and divided the posterior aspect of the oblique fissure, the arterial branches are identified. The anterior portion of the oblique fissure is divided as for left upper lobectomy and the arterial supply divided with an endostapler. The inferior pulmonary ligament is divided up to the level of the inferior pulmonary vein. The margins of the vein are clearly delineated and it is then divided. Bronchial vessels are clipped proximally and divided, and the lymph node chains are cleared off the medial and lateral aspects of the bronchus, which is divided at its base.
Segmentectomy-‘three-directional’ stapling technique
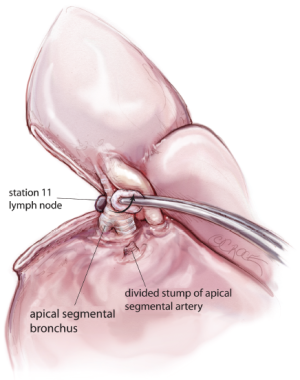
Apical segmentectomy of the lower lobe is a common procedure. In this article, I describe the technique of thoracoscopic apical segmentectomy using a ‘three-directional’ stapling technique. Having identified the PA in the oblique fissure and divided the posterior oblique fissure, the pulmonary artery is then prepared using blunt dissection by ‘dragging’ the lung tissue distally along the pulmonary artery until its bifurcation to apical and basal segmental branches is clearly seen. The apical segmental artery is divided using a vascular stapler (Figure 9). Once the apical artery is divided, the PA is pulled forward to reveal the bronchus intermedius posteriorly and its bifurcation to the lower lobe, i.e., apical and basilar segmental bronchi (Figure 10). The apical segmental bronchus is divided with a stapler, passed through the anterior access port. Lymph nodes are the cleared from the medial and lateral margins of the bronchus. The lower lobe is then retracted forward to exposure the posterior hilum. The lower lobe is further mobilized by dividing the inferior pulmonary ligament. The inferior vein is dissected free from surrounding tissue and the confluence of the apical and basilar segmental veins is developed by ‘pushing’ the lung tissue distally using a small pledget mounted on the tips of long dissecting forceps. The apical segmental vein is divided using an endoscopic linear stapling device (Figure 11).
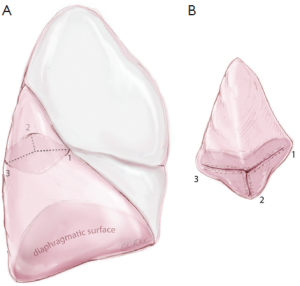
Finally, the apical segment is separated from the basilar tri-segments using a ‘three-directional’ stapling technique. It is clear that each lobe is a three-dimensional structure or pyramidal in shape. By simply compressing the lung tissue and dividing it using a heavy stapling device in one plane, not only is it not possible to achieve an anatomical segmentectomy, but also the staples may not be able to hold the thick lung tissues together, resulting in prolonged air-leak. It is important to first orientate the segment to its anatomical position. The ‘three-directional’ stapling technique requires the first stapler coming from the anterior access incision towards the distal limit of the apical segmental bronchus, compressing the interlobar surface with the anterior surface of the lobe; the second stapler coming from the posterior direction towards the distal limit of the segmental bronchus, compressing the lateral and posterior surfaces of the lobe; and the third stapler dividing the lung parenchyma medial and parallel to the apical segmental bronchus, hence completing the segmentectomy in three directions (Figure 12A). The final apical segmentectomy specimen should be pyramidal in shape with individually divided segmental artery, bronchus and vein (Figure 12B). All hilar and segmental level nodes relevant to the resected segment are excised. At mediastinal level either extensive sampling or lymphadenectomy is preferred.
Postoperative care
A size 32 Fr apical drain is placed through the mid-axillary line port site and is usually removed on the first postoperative day subject to a satisfactory chest radiograph and aerostasis. Patients are typically nursed on the general thoracic ward after immediate extubation. Analgesia is provided using a patient-controlled analgesia pump and a local anaesthetic paravertebral catheter. Early mobilization is strongly encouraged with the availability of physiotherapy seven days per week, and discharge as early as postoperative day 2 or 3 is often possible.
Comments
The posterior approach is a safe, reliable and reproducible approach to VATS lobectomy and segmentectomy. VATS has been shown to compare favorably with open thoractomy in terms of immediate post-operative recovery and is considered to be oncologically equivalent. Our cross-sectional survey on 838 thoracic surgeons worldwide showed that 95% of surgeons who performed VATS agreed with the CALGB definition of ‘true’ VATS lobectomy; 92% of surgeons who did not perform VATS were prepared to learn this technique, but were hindered by limited resources, exposure and mentoring (5). Majority of thoracic surgeons believed advanced VATS techniques should be incorporated into thoracic surgical training and for more standardized workshops to be made available. A recent consensus from 50 major minimally invasive thoracic surgeons showed that increased use of VATS techniques for lobectomy and segmentectomy would be highly desirable (1).
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Yan TD, Cao C, D’Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [PubMed]
- Cao C, Tian DH, Wolak K, et al. Cross-sectional survey on lobectomy approach (X-SOLA). Chest 2014. [Epub ahead of print]. [PubMed]