Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer
Introduction
First described over two decades ago, video-assisted thoracoscopic surgery (VATS) is now well established for the treatment of early stage non-small cell lung carcinoma (NSCLC) (1-6). Thoracoscopic approaches are preferred for many common thoracic procedures because of their predictable salutary effect on outcomes likely brought about by perioperative pain reductions. As widespread surgeon experience has grown with VATS, so has reliability. Low conversion rates are now commonplace despite the challenges associated with higher stage tumors and the tissue effects brought on by induction chemoradiotherapy. Tumors once thought unapproachable by thoracoscopic techniques are now frequently resected by VATS.
Video assisted thoracoscopic surgery offers many potential benefits compared to conventional muscle-splitting thoracotomy. Some established examples are decreased postoperative pain, diminished inflammatory response, decreased hospital length of stay (LOS), and faster recovery (7,8). By potentially allowing more patients to receive adjuvant chemotherapy compared to patients who undergo thoracotomy (9), VATS could potentially improve survival of patients with advanced NSCLC. Most large series examining results for VATS in lung cancer have been limited to early stage disease, and thoracotomy remains a staple for the surgical approach to locally advanced NSCLC (10). A variety of concerns regarding the completeness of oncologic resection, technical challenges, and potential safety concerns has limited the incorporation of thoracoscopy for more advanced stages of lung cancer. For patients requiring more extensive resection such as pneumonectomy and/or en bloc chest wall resection, thoracoscopic resection is even less common.
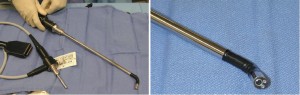
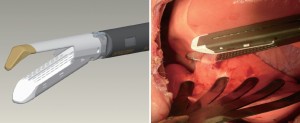
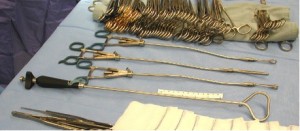
As previously demonstrated with many minimally invasive procedures, there is a learning curve with thoracoscopic anatomic resections (11). In general, this learning curve for advanced thoracoscopic cases has been aided by improved video, stapling, hemostatic, and retraction technologies. Excellent exposure is enabled by high-definition camera systems that allow viewing from various different angles (Figure 1). Endoscopic staplers have been modified to facilitate negotiation of delicate pulmonary vessels (Figure 2). Improved topical hemostatic technologies are useful when dealing with diffuse oozing from extrapleural or inflammatory dissections after induction therapy. Several companies now produce 5 mm low profile lung graspers (Figure 3). Up to 4 of these instruments can be placed through a single port incision to replicate the traction and counter-traction employed in open operations.
These technological advances have made thoracoscopic surgery safer allowing for the expansion of indications for thoracoscopic resection. This potentially increases treatment options for patients who otherwise may have previously been considered inoperable with thoracotomy. Here we will discuss key technical points/considerations for thoracoscopic resection for lobectomy in locally advanced non-small cell carcinoma, thoracoscopic pneumonectomy, and thoracoscopic en bloc chest wall resection.
VATS Pneumonectomy
VATS lobectomy for locally advanced NSCLCThoracoscopic lobectomy for locally advanced NSCL, though not as common as resection for early stage disease, has been reported (12). We consider tumors that are greater than 4 cm in diameter, T3 or T4 tumors (based on the American Joint Committee on Cancer, 7th edition), or tumors requiring neoadjuvant treatment to be locally advanced. Though a 4 cm tumor today may not seem advanced, original indications for VATS lobectomy during its evolution were for peripheral tumors less than 3 cm in diameter. Also, patients with tumors of this size were shown to have a survival advantage with adjuvant chemotherapy by Cancer and Leukemia Group B (CALGB) 9,633, suggesting more aggressive tumor characteristics in this subgroup of patients (13).
As surgical techniques have become refined and surgical instrumentation becomes more advanced, VATS has become our preferred approach for most forms of locally advanced NSCLC. We expect the demonstrated benefits associated with thoracoscopic resection for early stage NSCLC to translate to resections involving more locally advanced disease, provided overall tissue trauma remains less than for open procedures. Thoracoscopic lobectomy for advanced lung cancer can be performed safely with an acceptable morbidity and mortality (12). Perioperative complications were equal in patients undergoing thoracoscopic resection when compared to those having a thoracotomy, and a higher proportion of patients who underwent VATS resection were able to go on to receive adjuvant therapy. No difference was observed for disease-free and overall survival.
Thoracoscopic resections for locally advanced diseases pose challenges not encountered when performing typical VATS operations for early stage disease. Centrally located tumors and those in close proximity to major vascular structures, granulomatous or other lymphadenopathy, and post-neoadjuvant therapy effects increase thoracoscopic dissection complexity and difficulty. Tumors may invade the fissure requiring an en bloc segmental resection of an adjoining lobe. While completing the fissures first can be occasionally challenging, this ultimately leads to an easier, safer dissection of the lobe’s vascular and bronchial structures. For instance, such methods improve the exposure to vessels like the ascending posterior artery to the right upper lobes. They also make performance of a sleeve resection easier by removing other intervening anatomic structures. Practicing fissure division techniques on less challenging cases increases operator capability when confronted by difficult tumor anatomy.
With larger tumors and difficult anatomic dissections, having proper traction and counter-traction on structures is of paramount importance. Usually when difficulty arises during a dissection, one can find inadequate retraction angles to be the source of trouble. Proper retraction angles to facilitate safe dissection are now easier to create, and quickly adjust, with newer low profile 5 mm round shaft thoracoscopic instruments (Figure 3). Another concern regarding thoracoscopic approaches for technically difficult cases is that increased operative time, with prolonged general anesthesia, will prove detrimental to the patient and negate any proposed benefits of a thoracoscopic approach. In our previously reported data for VATS lobectomies for locally advanced NSCLC, median operative time for the thoracoscopic group was 231 minutes (96-574), compared to 202 minutes for the open group (105-317) (12). We feel these operative times are not unreasonable. Previous concerns regarding the duration of general anesthesia exposure and its impact on patient results may not be as relevant if the operation can be completed with VATS. For instance, increased resources like extended operative times with thoracoscopy may be justified if avoiding thoracotomy in a frail patient reduces the need for prolonged convalescence.
VATS Pneumonectomy
While thoracoscopic lobectomy is an established operation, the safety and potential benefits of thoracoscopic pneumonectomy are uncertain. Thoracoscopic pneumonectomy utilizing a traditional 3-incision VATS approach has been described (14), and recently single-port pneumonectomy has recently been reported (15). Whether the well-defined benefits noted with thoracoscopic lobectomy translate to thoracoscopic pneumonectomy is uncertain.
We reported the intention-to-treat results from our modest experience and demonstrated that thoracoscopic pneumonectomy is a safe alternative to open pneumonectomy. Results were equivalent to those patients undergoing pneumonectomy using a standard thoracotomy approach (16). Median blood loss was equal, as well as median ICU length of stay and hospital length of stay. Operations were longer, and though operative blood loss was similar, transfusions were increased in the thoracoscopic pneumonectomy group. Major complications were similar for both groups. Though a significant conversion rate of 25% was noted, there were no significant differences in any postoperative complications between the thoracoscopic group and the group requiring emergent conversion (16). When long-term survival was examined in patients undergoing thoracoscopy versus thoracotomy for elective pneumonectomy, results were equivalent (17). Table 1 summarizes technical challenges associated with performing VATS pneumonectomy and solutions for overcoming them.
| Table 1 Technical challenges and hurdles associated with VATS pneumonectomy | |
| Challenge | Solution |
| Division of the ribs/bone | Thoracoscopic bone shears (Sofamor-DanekTM) exist to facilitate rib division. Standard bone cutting tools can be used for division near utility incision |
| Chest wall soft tissues | Standard thoracoscopic cutting energy devices allow for division of muscle and the neurovascular bundle |
| Location of tumor invasion related to standard VATS incisions | Utility port location may need to be altered in some cases. Moving it towards the anterior thorax may aid in dissection as well as extraction of the specimen (due to the wider intercostal space) |
| Pancoast Tumors/Spine Invasion | Combined approach with surgical spine team utilizing posterior spine approach, followed by VATS approach to lung resection is feasible |
| Extraction of the specimen | Sturdy 8 by 10 in Nylon extraction sac. Orientation of the rib block perpendicular to the extraction site while delivering the ribs through the port first. An alternative site for extraction may be necessary |
VATS Resection with en bloc chest wall resection
Performing thoracoscopic en bloc chest wall resection at the time of lobectomy constitutes another area of potential expansion for VATS techniques. As noted with the expansion of techniques and indications for VATS lobectomy and pneumonectomy, improved instrumentation has made thoracoscopic chest wall resection feasible. Our initial experience indicates that it is technically safe, but large single center series are not yet available to define refined techniques on this subject.
Technical maneuvers already exist that enable minimally invasive approaches to the chest wall for tumors and other pathology related to thoracic bone anatomy. Special bone cutting tools have been developed by surgeons in other subspecialties including minimally invasive devices to procure bone grafts (18). For chest wall resections in which a large chest wall defect will be expected, minimally invasive options now exist. Muscle flaps commonly required in complex thoracic chest wall resections for coverage like the latissimus dorsi have been mobilized by videoendoscopy (19). Few reports have good comparison groups when evaluating postoperative recovery effects. Advantages for VATS over open thoracotomy may be counterintuitive in the context of chest wall resections, however pain physiology theories exist to explain why pain would be potentially less with approaches that “on the surface” are less invasive (20). This is because the innervation for that area is removed by the resection and the remainder of the wound space stimulation, which in its totality induces chronic pain, is minimized.
Patient selection criteria for whom to apply a thoracoscopic approach for chest wall resection are not clearly defined and will be influenced by surgeon experience. As was our approach when expanding indications for VATS lobectomy and pneumonectomy, our preferred group for extending thoracoscopic indications includes those frail patients expected to have the most difficulty with thoracotomy. This similar ideology has been applied by another group for less invasive laser resection for T3 chest wall tumors in 10 patients who had poor pulmonary function (21). Since improved exposure begets operative precision, we are confident that surgical planning by adding an internal VATS view of rib invasion before or during thoracotomy is an improvement over the traditional reliance on normal-appearing external landmarks.
Because the experience with thoracoscopic approaches for tumors is limited largely by the infrequent nature of suitable cases, validating long terms results will be challenging. This will require substantial time and a cooperative framework to determine if there are any long-term advantages for a thoracoscopic approach to tumors with chest wall invasion, or even primary chest wall tumors. In the meantime, knowledge of the techniques that will be useful for experienced VATS surgeons who encounter complex anatomic situations will continue to expand and be refined. Table 2 summarizes technical considerations for thoracoscopic chest wall resection.
| Table 2 Technical challenges and considerations for en bloc chest wall resection | |
| Challenge | Solution |
| Concern regarding the possibility of stapler induced injury or stapler misfire when coming across and dividing main pulmonary artery with limited vascular control |
Guiding stapler with red rubber “leader” facilitates safer passage across the main pulmonary artery |
| Safety of pulmonary artery dissection | Dissection onto the mainstem bronchus when performing mediastinoscopy/Transcervical Extended Mediastinal Lymphadenectomy (TEMLA) will make vascular dissection easier/safer at time of VATS resection |
| Tissue coverage for the bronchial stump | Creation of a pericardial fat pad and/or pleural flap is safe Thoracoscopic intercostal muscle flap is also feasible |
| Getting proximal division point on the main stem bronchus, especially with a left pneumonectomy |
Lung retraction instrumentation now allow for aggressive retraction for proximal division of the bronchus Use of Transcervical Extended Mediastinal Lymphadenectomy (TEMLA) before resection |
| Technical consideration of retracting the whole lung when dividing the mainstem bronchus |
A laparoscopic adjustable liver retractor (Snowden-Pencer Diamond-Flex) placed around the main stem bronchus allows for retraction of the entire lung (Figure 4) |
| Removing the specimen from the chest cavity | Larger 8 inch by 10 inch Nylon extraction sac Facilitates removal of the entire lung |
Summary
Initial fears regarding the oncologic equivalence of the thoracoscopic and open techniques for resecting NSCLC have not been realized. Reported data to date indicate that even in advanced NSCLC requiring pneumonectomy, the overall and disease free survival are equivalent for patients undergoing VATS versus thoracotomy. Furthermore, these results have occurred during a time where the complex procedures are still in a relatively early stage of refinement and we sense that results will improve as we make adjustments to speed the operations and further reduce conversions and complications. VATS lobectomy for early stage disease produces oncologically similar results with open techniques, and long term studies will determine if the same hold true for more advanced case. Early indications are favorable. This finding is in accordance with others who have hypothesized that the reduced inflammatory response associated with thoracoscopy may be associated with equivalent or even improved long-term survival (22,23).
VATS lobectomy, pneumonectomy, and chest wall resection for advanced lung cancer can be performed safely with an acceptable mortality rate. VATS offers the benefit of increased tolerance for adjuvant therapy so if high VATS reliability is achieved, it may be reasonable someday to consider resection first for some patients who currently undergo induction chemoradiotherapy for their disease. The low morbidity of VATS reported for early stage lung carcinoma, though not definitively proven for advanced stage NSCLC, will be expected as experience builds.
Further analyses of outcomes for thoracoscopic resection of advanced stage disease are ongoing. This is particularly important given the large number of frail patients with advanced stage disease who require multimodality therapy, which can be difficult to tolerate. Conversions, though increased in frequency, are not associated with a significant change in short-term or long-term outcomes. Continued improvements in instrument technology and surgical technique will only continue to expand the possibilities for minimally invasive pulmonary resections, as well as those for primary chest wall tumors.
Acknowledgements
Disclosure: Mark W Hennon has no financial disclosures to report. Todd L Demmy, has received a one-time compensation for intellectual property as well as educational grants from Covidien.
References
- Hartwig MG, D’Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101.
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78.
- Cheng D, Downey RJ, Kernstine K, et al. Video-Assisted Thoracic Surgery in Lung Cancer Resection: A Meta-Analysis and Systematic Review of Controlled Trials. Innovations (Phila) 2007;2:261-292.
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25.
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6.
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5.
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5.
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7.
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250.
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1.
- Ng T, Ryder BA. Evolution to video-assisted thoracic surgery lobectomy after training: initial results of the first 30 patients. J Am Coll Surg 2006;203:551-7.
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6.
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51.
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4.
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc 2012. [Epub ahead of print].
- Sahai RK, Nwogu CE, Yendamuri S, et al. Is thoracoscopic pneumonectomy safe? Ann Thorac Surg 2009;88:1086-92.
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6.
- Steffen T, Downer P, Steiner B, et al. Minimally invasive bone harvesting tools. Eur Spine J 2000;9:S114-8.
- Martinez-Ferro M, Fraire C, Saldaña L, et al. Complete videoendoscopic harvest and transposition of latissimus dorsi muscle for the treatment of Poland syndrome: a first report. J Laparoendosc Adv Surg Tech A 2007;17:108-13.
- Demmy TL, Nwogu CE, Yendamuri S. Thoracoscopic chest wall resection: what is its role? Ann Thorac Surg 2010;89:S2142-5.
- Mineo TC, Ambrogi V, Pompeo E, et al. En bloc minimal laser resection for T3-chest wall lung cancer in patients with poor pulmonary function. Chest 1996;110:1092-6.
- Ng CS, Wan S, Hui CW, et al. Video-assisted thoracic surgery lobectomy for lung cancer is associated with less immunochemokine disturbances than thoracotomy. Eur J Cardiothorac Surg 2007;31:83-7.
- Ng CS, Wan S, Hui CW, et al. Video-assisted thoracic surgery for early stage lung cancer - can short-term immunological advantages improve long-term survival? Ann Thorac Cardiovasc Surg 2006;12:308-12.






