Mechanical circulatory support devices as destination therapy—current evidence
Background
Nowadays, heart transplantation (HTX) is the treatment of choice for selected patients with end-stage heart failure, with over 85,000 performed procedures worldwide during the last four decades. On average, more than 4,000 heart transplants are carried out every year, in over 249 centers worldwide (1). However, even significant scientific progress and numerous innovations within the field cannot solve the two current eminent problems of cardiac transplantation—a continuously increasing number of heart failure patients, and a dramatic decrease in suitable donor organs. In 2013, only 299 hearts were donated and 297 HTXs were performed throughout Germany. This has resulted in a major imbalance between supply and demand, and around 20% of patients die whilst being on the waiting list for a heart transplant (currently around 904 patients in Germany) (2,3). As a consequence of persistent donor organ shortage, there has been a growing interest for alternative strategies, in particular mechanical circulatory support (MCS) not only as a bridge to transplantation (BTT), but also as a destination therapy (DT). Improved results and increased applicability and durability of left ventricular assist devices (LVADs) have established this treatment option as an alternative for end-stage heart failure patients. Medical treatment with blockade of the neuro-humoral pathway, inotropic support and cardiac resynchronization is only able to improve clinical symptoms of the patients in the short term and has resulted in a disappointing survival rate of just 10-30% (4). Already, back in 2001, the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial first demonstrated that implantation of LVADs as DT can provide better survival compared to any other known medical treatment in patients with end-stage heart failure who were ineligible for transplantation (5). The authors recommended at that time that appropriate selection of candidates and timing of LVAD implantation are critical for improved outcomes of DT. In a subsequent study, patients with advanced heart failure who were referred for DT before major complications of heart failure developed displayed the best chance of achieving an excellent one year survival with LVAD therapy (6).
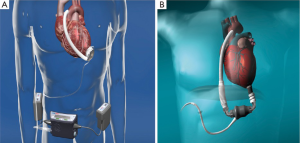
Since then, subsequent studies investigating various types of LVADs have supported the benefits of LVAD implantation for advanced heart failure where HTX is deemed unsuitable (7). So far, three generations of LVADs have been on the market. The first generation of implantable ventricular assist devices (VADs) were pulsatile, volume-displacement pumps such as the HeartMate XVE in 1998 (Thoratec Inc.; Pleasanton, Calif, US). These devices had numerous limitations such as a large volume requirements and the need of excessive surgical dissection for device implantation. During the following decade, significant improvements in pump design resulted in a new generation of LVADs—smaller and non-pulsatile continuous-flow rotary and axial blood pumps (second- and third-generation LVADs). These newer pumps represent a milestone for LVAD-development and provide even better patient-outcomes, which have enabled currently available LVADs such as the Heart Mate II (HM II) (Thoratec Inc. USA) (Figure 1), the Berlin Heart Incor (Berlin Heart AG, Germany), DuraHeart (Terumo, USA) and the HeartWare Ventricular Assist System (HVAD) (HeartWare Inc. USA) (Figure 1) to become the standard of care in specialized centers (8).
Indication for DT
DT refers to patients who are not eligible for HTX and in whom an LVAD is the only effective option to treat terminal heart failure. The sixth INTERMACS report showed that from a total of 10,542 MCS implantations, nearly half of patients from 2011-2013 (41%) received an MCS as DT (9). In 2012, the European Society of Cardiology (ESC) published guidelines for the use of LVAD therapy which are shown in (Table 1). In general, these criteria for LVAD implantation were based on the patient selection criteria from the REMATCH trial (10). These included patients who have New York Heart Association class IV symptoms for at least 60 days under optimal heart failure therapy, or need inotropic support for heart failure treatment; display a left ventricular ejection fraction (LVEF) under 25%; have a peak oxygen consumption of 10,11). Patients selected for DT usually have contraindications for heart transplantation, such as age >70 years, malignancy within the past five years, comorbidities such as insulin-dependent diabetes mellitus with end-organ damage, chronic renal failure, drug abuse, severe obesity or fixed pulmonary hypertension with a transpulmonary gradient of above 15 mmHg and vascular resistance >6 Wood Units (12). The best time point for implantation of an LVAD is still under debate, though it is well documented in the literature that patients with high INTERMACS categories have the best outcome with LVADs, including those assigned to DT (8,9). In general, the decision regarding when to implant an LVAD as DT should be based on published scientific evidence and INTERMACS values (Table 2). In addition to profound clinical experience by the responsible physician or surgeon, it requires a holistic evaluation of individual clinical patient parameters and preferences such as quality of life and tolerance of adverse events (13). Ultimately, the patient must decide between LVAD implantation at a later timepoint and lower INTERMACS level, running the risk of rapidly deteriorating heart failure in the meantime, or earlier LVAD implantation at a higher INTERMACS level, with the risk of complications associated with LVAD therapy.

Full table

Full table
The intention of the patient’s LVAD treatment, whether it be BTT or DT, is often assigned prior to device implantation. However, Teuteberg et al. emphasised that the possibility of a heart transplant during LVAD support changes continually over time. In an analysis of 2,816 patients enrolled in the INTERMACS database, he showed that 43.5% of patients who were initially implanted with BTT intent were no longer listed for cardiac transplantation at two years after LVAD implantation. At the same time, nearly 15% of patients assigned to DT were considered for transplant. Therefore, the most common pre-implant strategy may be a bridge to candidacy (BTC), in particular as the implant strategy also forecasts patient outcome. The two year survivals of patients supported for BTT, BTC, and DT were 78%, 70% and 61%, respectively. Rapid changes in patients’ nutritional status, functional status, end-organ function, and adherence after LVAD can affect transplant candidacy and post-transplant survival. Therefore, there is a need for continued re-evaluation of the implant strategy and indication (BTT vs. DT) (7).
Outcomes of DT
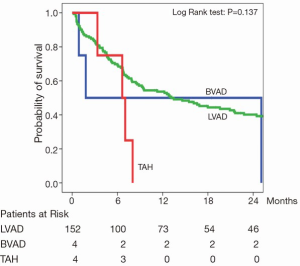
Many efforts have been made to optimize technical design, flow characteristics and durability of LVAD systems to enable long term support (Figure 2). A particularly important technical milestone was the implementation of the continuous flow left ventricular assist devices (CF-LVAD). In 2009, Slaughter et al. highlighted in a randomized multicentre trial with 200 DT patients, that intended treatment with CF-LVAD, in this case the Heart Mate II device, resulted in a significant better one- and two-year survival (68%, 58%) as compared to a pulsatile Heart Mate I LVAD (55%; 24%). Remarkably, 18 patients were switched from pulsatile to CF-LVAD during the follow up period. Although the functional status in both groups was improved by LVAD therapy, major adverse events, such device-related and non-device-related infections were significantly reduced in the CF-LVAD group. However, the incidence of strokes did not differ significantly between the two groups (14). Data from our own institution, the Heart and Diabetes Center NRW in Bad Oeynhausen, also shows better outcomes for patients treated with CF-LVADs compared to compared to the patients with biventricular pulsatile VAD’s or patients on total artificial hearts (Figure 3). Furthermore, Rogers and colleagues demonstrated additional benefits for functional NYHA status and quality of life in a retrospective analysis of BTT and DT Heart Mate II patients in the above mentioned trial (15). In the DT (CF-LVAD) group, NYHA functional class improved from class IV to class I-II in 80% of patients at between six and 24 months (15). Recently, results of the post-FDA-approval study with the Heart Mate II for DT were reported (16). This study was a prospective evaluation of the first 247 consecutive Heart Mate II patients who underwent implantation after FDA approval of the device, and who were preoperatively identified for DT between January and September 2010. This cohort was compared to a historic patient group of DT Heart Mate II patients (n=133) of the pivotal trial. Baseline characteristics did not differ between both groups. Heart Mate II was implanted at INTERMACS levels 1 and 2 in 45% of patients, and at INTERMACS levels 2-3 in 28% of patients. Survival at one and two years was 74±3% and 61±3% in the post approval respectively, and 68±4% and 58±4% in the pivotal trial group, respectively. According to the INTERMACS levels, patient survival was worse at level 1-2 and best at INTERMACS level 4-7. Comparing adverse event rates, the authors found a general trend towards lower rates in the post approval cohort, including bleeding, device related infections, ischemic stroke and pump exchange for all reasons, but not for hemolysis, which had an increased incidence. The authors concluded that treatment with Heart Mate II for DT is superior to medical therapy in patients with terminal heart failure, but also raised a word of caution regarding the extension of LVAD implantation in ‘less sick’ patients, as a further reduction of adverse event rates would be essential to ultimately reach this goal (16). Recently, results of the investigator initiated post-market registry to evaluate the HeartWare Left Ventricular Assist System (ReVOLVE) were published. From February 2009 to December 2012, 314 HeartWare implants were enrolled into this registry. The primary outcome was defined as survival to transplant, successful recovery with the device being explanted, or ongoing continued HeartWare system support. Duration of HeartWare support ranged from 1 to 1.057 days and patient selection differed in the current study in that aspect that patients were older, and there were a higher percentage of females and patients with idiopathic cardiomyopathies compared to the ReVOLVE cohort (17). HTX was performed in 56 patients (22%), explant for recovery occurred in three patients (1%), 43 died whilst on support (17%), and 152 (60%) remained on the device. Successful support of patients with HeartWare was 87% at six months, 85% at one year, 79% at two years and 73% at three years. Adverse event rates were low and comparable to or even improved, compared to the CE-Mark-Trial. Complications included bleeding (28%), RV failure (9%) and driveline infections (6%) (17).
Non device related problems
Right ventricular (RV) failure
The failing right ventricle is the burden before and after MCSD implantation, and influences the outcome after LVAD implantation for DT. Therefore, the major problem during LVAD treatment is the question of whether the frequently impaired RV function is recovering after unloading of the left ventricle. Beyersdorf et al. published their experience with LVADs in patients with fixed pulmonary vascular resistance (PVR) for DT (18). A significant reduction in PVR was observed after several months of support. In addition, fixed PVR was successfully reversed with pulsatile and non-pulsatile VADs (18,19). Reduction of PVR occurred even in patients with markedly elevated PVR (>7 WU), and strikingly, pulmonary hypertension (PHT) did not return after HTX in these patients. Post-transplant survival of this cohort was comparable with that of patients without prior PHT. The observation that unloading of the left ventricle by pulsatile and nonpulsatile VADs is able to reverse previously “fixed” PVR was subsequently confirmed by other groups (20,21). Atluri and colleagues identified central venous pressure >15 mmHg, severe echocardiographic RV dysfunction, preoperative ventilation, severe tricuspid regurgitation and a heart rate >100 as the major predictors of right ventricular failure before LVAD implantation, necessitating biventricular support (22). Device type has a large impact on survival rates, and the INTERMACS registry reports on an excellent one year survival of 81% after implantation of a continuous flow LVAD, compared to a reduced survival of 65% with a pulsatile LVAD. Therefore, continuous flow LVADs should primarily be used in DT to improve outcomes (9), as biventricular support has a significant worse outcome with survival rates of only 57% after one year (7). In terms of supporting only the left ventricle, there is up to 50% incidence of right ventricular failure after LVAD implantation, and as a consequence, perioperative mortality and morbidity rises up to 19% to 43% (5,6). Data from our own center show that temporary percutaneous right heart support by extracorporeal means and LVAD implantation is superior compared to first line implantation of a biventricular VAD during the first 48 hours after post-procedural RV failure (7 vs. 0 pts; P=0.005). Follow up after six months surprisingly revealed no significant difference in mortality between the two groups, and significantly more patients suffered from multiple organ and RV failure in the LVAD cohort. Thirty-seven patients required a delayed right heart support after initial LVAD implantation (23).
Device related problems
Acquired von Willebrand Syndrome and gastrointestinal bleeding
A recent report from Segura et al. in 2013 described histologic changes within the aortic wall driven by continuous-flow LVAD support in 11 patients after LVAD support, at between 87 and 580 days. Aortic wall samples before and after continuous flow LVAD implantation showed smooth muscle and elastic fiber degeneration, medial fibrosis and also arteriosclerosis (24). However, long-term cardiovascular changes from non-pulsatile flow LVADs have not yet been investigated in detail and their effect is currently unclear (25). In addition, the phenomenon of acquired von Willebrand Syndrome during long term CF-LVAD therapy is not yet completely understood, but its pathology is reversible after HTX (26). Meyer et al. found a decrease of up to 34% in the high molecular multimers, independent of device type (Heart Mate II or HeartWare). These molecules play an important role in the primary hemostasis and result in reduced platelet activity and aggregation. Although causes of bleeding during LVAD therapy are multifactorial, several other groups have confirmed that acquired von Willebrand Syndrome has developed in patients with long term CF-LVAD due to the loss in high-molecular-weight von Willebrand factor (vWF) multimers (27-30). Moreover, the von Willebrand antigen (vWF:AG) and ratio of vWF to collagen binding activity (vWF:CB) may be potential markers of disease, as the mean vWF:AG and vWF:CB were significantly higher in patients with the Heart Mate II compared with the HeartWare. In addition, lower pump speed in HeartWare patients results in reduced loss of large multimers, whereas speed does not affect the vWF profile in Heart Mate II devices. This subtle difference in vWF profiles between the devices and patients, however, does not seem to correlate with overall bleeding complications (27).
Gastrointestinal bleeding is one of the major adverse events after CF-LVAD implantation, with an incidence of 19-40% (31,32). It is a leading cause for readmission within the first six months after LVAD implantation (33). Its main causes include arteriovenous malformations, ‘over anticoagulation’, and the acquired von Willebrand Syndrome. However, the rate of gastrointestinal bleeding observed in the ReVOLVE study was quite low, only 5% (17). In contrast, in the more rigorous clinical trial setting of U.S. BTT and continued access protocol (CAP) studies, the incidence of gastrointestinal bleeding was around 10% in 1,496 post-trial patients receiving a Heart Mate II device (34) and 12.7% in HeartWare patients (35,36). One explanation for this may be the differences in pump design, as Heart Mate II has an axial pump design, whereas HeartWare uses a centrifugal pump. In addition, there is usually a much stricter patient selection within a trademark study design such as the REVOLVE study and stricter patient management. Other single-center studies have identified age, ischemic cardiomyopathy, hypertension, body mass index (BMI), albumin, cardiopulmonary bypass time, and a history of gastrointestinal bleeding as pre-operative risk factors for bleeding post-LVAD implantation (31,37).
Driveline fracture, pump- and driveline infection
A partial or complete infection involving the device is a serious complication and often associated with an adverse outcome. In particular, infections involving the driveline influence long-term outcomes, and technical problems with the device may ultimately require a pump exchange. In a recent study, Moazami et al. reported that 6.4% of 1,128 patients with an implanted HMII needed a pump exchange after a mean support time of 568±535 days. Reasons for pump exchange were infections in 0.6% of patients and lead damage in 3%. Out of all the patients who underwent a pump exchange, 6.5% died in the first 30 days postoperatively and 30% died during the first postoperative year, while 65% remained on continuous support and 5% were transplanted (38). In a further study, Schaffer et al. compared 86 CF-LVADs with 47 pulsatile flow LVADs implanted between 2000 and 2009. The authors could demonstrate that Staphylococcus species were responsible for 50% of driveline and bloodstream infections (39). In an interesting study by Sinha et al., 86 patients having received a LVAD were matched to 50 transplanted patients by comorbidities, age, sex and transplant date. Freedom from peri-transplant and post-transplant infections was compared at six months after transplant and survival was compared at three years. In this cohort, 44 patients (51%) were successfully discharged home on LVAD support, and 61 (71%) were transplanted. Interestingly, the authors demonstrated that a high incidence of infection during device support did not impact pre-transplant or post-transplant mortality, post-transplant infectious rate, or overall survival (40). Active infections at transplant also did not significantly influence six-month mortality. Although LVAD recipients had a significantly lower freedom from infection than the control heart transplant group, three-year survival did not significantly differ [79% (LVAD) vs. 87% (control)] (40). Finally, in a large database analysis, Kalavrouziotis et al. investigated 12,969 worldwide implanted Heart Mate II LVADs. A percutaneous lead dysfunction occurred in 1198 devices (9.2%) over a cumulative support period of 13,932 patient-years. As expected, lead failure was mostly localised to the external part of the cable (87.2%) and could be managed in 76% of the cases by clamshell reinforcement of the external connector strain or by tape or silicone cable reinforcement. Mortality and significant morbidity, including pump exchange, urgent transplant or a more extensive lead repair occurred in 2.3% of all implanted devices (41).
Thromboembolic events
Another frequent complication of long term LVAD support in the context of DT are thromboembolic complications resulting in neurologic sequelae (42). Although modern CF-LVADs such as the Heart Mate II have a significantly reduced risk of thromboembolic events and strokes compared to earlier devices, postoperative neurologic sequelae are still an important cause of morbidity and reduced quality of life (43,44). Only a few studies have addressed this problem and evaluated risk factors for stroke and thromboembolic events in CF-LVAD patients, which include a history of cerebrovascular accident, low serum sodium, and low serum albumin (45), high right atrial pressure, enlarged right ventricular end-diastolic dimension and preoperative atrial fibrillation (46,47). Recent results for 9,372 patients from the INTERMACS database suggested significant improvements for neurologic dysfunction with CF-LVADs (11). Morgan et al. reported in a series of 100 Heart Mate II patients (35 patients/DT and 65 patients/BTT) a total of 12 patients suffering strokes (12.0%). These included seven BTT patients and five DT patients, resulting in an overall incidence of stroke of 10.8% (7/65) for BTT patients and 14.3% (5/35) for DT patients. The etiology of strokes was embolic in four patients and hemorrhagic in eight. Median duration of support at the time of stroke was 340.5 days (range: 4-1,161 days). Patients with stroke had a significantly higher incidence of diabetes, history of previous stroke, and use of aortic cross-clamping with cardioplegic arrest during LVAD implantation compared with patients without neurologic events. At the time of stroke, 11 of the 12 patients were on warfarin, with a sub-therapeutic INR in all four patients with embolic strokes and supra-therapeutic INR in four of eight patients with a hemorrhagic stroke. Regarding antiplatelet therapy, 11 of the 12 patients were on 81 mg of daily aspirin at the time of stroke, and the effect of a stroke had a profound impact on survival, as mortality within 30 days of stroke was 25.0%. Among the nine surviving patients, two were transplanted, six were on ongoing LVAD support and one died 17 months after the stroke. Unsurprisingly, a Cox multivariate logistic regression analysis revealed that diabetes (OR 6.36; P=0.029), aortic cross-clamping with cardioplegic arrest (OR 4.75; P=0.025), duration of support (OR 1.00; P=0.008), and INR (OR 4.42; P=0.020) were independent predictors of stroke (48).
Pump thrombosis and pump exchange
Pump durability is particularly important for patients assigned to DT. Pump function and reliability are important for patients assigned to DT. This is related to the interaction between implantation techniques and anatomical characteristics of the patient. In addition, the diagnosis of LVAD thrombosis is challenging, as it involves a combination of clinical symptoms, serologic markers, imaging studies and changes in device power consumption (49). Shah et al. investigated 241 patients with either centrifugal CF-LVAD or axial CF-LVAD, implanted between 2000 and 2012. The results suggested that LDH may be a more sensitive marker for hemolysis than serum free hemoglobin in patients with a continuous flow device, and concluded that LDH is superior in detecting device thrombosis (50). Boyle et al. reviewed 469 patients enrolled in the BTT arm of the US Heart Mate II pivotal trial and reported a very low incidence of pump thrombosis of only 0.9% in patients who received warfarin and antiplatelet therapy (51). Although mortality on VAD support continues to decrease, morbidity due to device thrombosis is becoming more apparent, particularly in DT patients as a consequence of long-term support. Furthermore, suspected or confirmed thrombosis was one of the most common indications for device exchange in an analysis of 1,128 patients, of whom 72 underwent pump exchange between 2005 and 2010 (52). Operative mortality for pump exchange at 30 days was 6.5%, and 65% of the patients were alive two years after exchange. However, considering that one-year survival after the first continuous-flow LVAD implant is nowadays about 80%, about 65% after a second implant, and only 50% after a third implant, prevention of pump malfunction and pump thrombosis becomes crucial (52). In this regard, an interesting observation was reported by Starling et al., who had observed an incremental increase in Heart Mate II pump thrombosis in three large LVAD centers in the US since 2011. Among 895 Heart Mate II patients, 72 confirmed pump thromboses were observed in 66 patients, and additionally in 36 patients a pump thrombosis was suspected. Remarkably, the occurrence of confirmed pump thrombosis increased rapidly after March 2011, from 2.2% at three months after implantation to 8.4% by January 2013. The same trend was observed at all three implanting institutions and for all operating surgeons (53). Similarly, an INTERMACS database analysis demonstrated an increase in pump thrombosis from 2% before May 2011 to 5% from May 2013 onwards (53). One critical point was that the anticoagulation protocol was changed during the study and therefore could have resulted in the higher incidence of pump thrombosis (54). Another explanation may be the deposition of fibrin and denatured protein in the proximity of the inflow bearing, followed by heat generation with increasing shear stress on the red cells. If the deposition of fibrin and denatured protein becomes large enough, this could impair the pump’s ability to unload the left ventricle. The “bearing–fibrin” deposition theory could therefore explain the hemolysis that develops as thrombus deposition begins (53).
Rhythm disturbance
Ventricular arrhythmias are common in patients with CF-LVADs and there have been reports of patients surviving several months with ventricular fibrillation (VF) on LVAD support (55,56). Current theories suggest that LVADs are arrhythmogenic by introducing new areas of scarring or by altering gene expression of ion channels possibly involved in arrhythmogenesis (57). Cantillon et al. demonstrated that the presence of an ICD was associated with improved survival in patients with LVADs (58). In another study, 94 patients were enrolled after long term CF-LVAD, of whom 77 had an ICD and 17 did not. Twenty-two patients had a ventricular arrhythmia >30 days after LVAD implantation, and the authors showed that pre-operative ventricular arrhythmia was the major predictor of post-operative rhythm disturbances (4.0% vs. 45.5%; P59). No patients discharged from the hospital without an ICD after CF-LVAD implantation died during 276.2 months of follow-up. The authors concluded that patients with pre-operative ventricular arrhythmias are at risk of recurrent rhythm disturbances during CF-LVAD support and should have an active ICD therapy to minimize this risk. Patients without pre-operative history of rhythm disturbances are at low risk and may not need active ICD therapy (5).
Recovery from LVAD
The possibility of weaning from the device should be considered, especially in DT patients. Despite initial encouraging attempts to wean patients from LVADs, the percentage of patients undergoing LVAD explantation for myocardial recovery remains very low (5-24%) and is only reported in small case series (60-62). Recent data from the INTERMACS registry report a decreasing percentage of a Bridge to Recovery (BTR) strategy for LVADs over the last years, below 1% for 2013. The highest recovery rate (73.3%) in patients with IDCM was published by Birks and colleagues (63) using pulsatile-flow LVADs and treatment with clenbuterol. However, these data could not be reproduced by any other group. In a retrospective analysis of their MCS population from 1992 to 2009, Krabatsch et al. demonstrated that in 44 out of 387 patients with idiopathic dilated cardiomyopathy, LVAD explantation due to myocardial recovery was possible. Throughout this study, the initial weaning incidence was around 10.8% and the institutional weaning rate was 8.8%. The remaining 343 patients did not reach the institutional weaning criteria. In this trial, patients on pulsatile device had a threefold higher chance of weaning from an LVAD than with a non-pulsatile device. Younger patients had a significant better weaning rate than older patients (37.9±18.7 vs. 52.4±14.2 years) (64). Although recovery after LVAD implantation is rare, Patel et al. demonstrated in a small single-arm prospective study with 21 patients that the combination of maximal neuro-hormonal blockade with heart failure medication and continuous-flow LVAD resulted in significant reverse remodelling. This process included a decrease in left atrial volume index and left ventricular internal diastolic diameter, and an increase in left ventricular ejection fraction. Although LVAD support could be weaned in only three patients, this study clarified that after LVAD implantation, optimization of the heart failure medication is necessary and should be continued in any case (65).
Risk scores, pre- and post-operative care and costs
Rosenbaum et al., using the Seattle Heart Failure Score (SHSF), investigated whether LVADs can be implanted in selected patients over the age of 65 years with acceptable survival compared with published outcomes in younger patients. In a single center study, he analyzed a cohort of 64 patients above 65 years with a CF-LVAD for BT or DT from 2005 to 2012. The patients showed a median survival of 1090 days and a survival rate of 85%, 74%, 55% and 45% at six months, one, two and three years. The observed survival was better than the SHSF calculated survival for both groups. Flint et al. investigated the association between pre-operative health status, as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), and survival and hospitalization after LVAD in 1,125 clinical trial participants who received the Heart Mate II as DT (n=635) or BT (n=490). He demonstrated that the KCCQ score among survivors and non survivors did not correlate with overall, 30 and 180 day mortality after Heart Mate II implantation. He concluded that the preoperative health status only has a limited association with outcome after LVAD implantation (66).
Several studies have evaluated the long-term outcomes and costs associated with LVAD therapy. Nearly ten years ago, the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) study randomly assigned patients ineligible for transplant to treatment with an LVAD or optimal medical management (OMM). The LVAD patients had survival rates of 52% at one year and 23% at two years compared with 25% and 8% in the optimal medical management arm (5). Mean costs for the implant-related hospitalization was $210,187 at that time (67). A follow-up cost-effectiveness analysis based on the REMATCH trial published in 2004 concluded that the incremental cost-effectiveness ratio (ICER) was $802,700 per quality-adjusted life year (QALY). As centers gained more experience with patient selection, device implantation, and postoperative management, costs for the initial implant hospitalization decreased. Along this line, Miller et al. presented cost data from a cohort of patients implanted with a pulsatile flow LVAD after completion of the REMATCH trial in select high-volume centers and demonstrated that the mean cost for implantation decreased to $128,048 (68). In a contemporary review of six pulsatile LVAD studies, Clegg et al. reported a cost per QALY of $341,573 and cited a potential improvement in LVAD cost-effectiveness with the introduction of continuous-flow devices (69). Slaughter et al. again analyzed the cost for 83 CF- and 52 PF-LVAD patients. As suspected, the hospital length of stay and in-hospital mortality was lower in the CF-LVAD cohort and as a consequence the inflation-adjusted hospital costs were significantly lower for CF- compared to PF-LVAD patients (mean: $193,812 vs. $384,260; P70).
Conclusions and future perspectives
As a consequence of organ donor shortage and an aging population, HTX is not an option for every terminal heart failure patient nowadays. Currently, DT with an LVAD is often the only therapeutic alternative for these patients, with the Heart Mate II and the HeartWare devices being the most frequently implanted CF-LVADs worldwide for DT. Despite the decreased incidence of pump thrombosis, driveline infection and thromboembolic events in recent years, these complications still significantly contribute to the morbidity and mortality of this therapy. Therefore, the next steps for improving the devices must address these problems.
Further miniaturization of the devices will lead to an easier and less traumatic implantation technique. Improved coating of the foreign surfaces and pump design will further reduce pump thrombosis and clot formation during long-term support, enabling less rigid anticoagulation protocols. One crucial step towards prevention of device-related infections would be the avoidance of a driveline by transcutaneous energy transfer (TET). In addition, the next generation of current devices such as the Heart Mate III will be able to mimic pulsatile flow through rapid changes of the pump speed, with potential benefits, as the long term effects of continuous flow devices on the vasculature and organ perfusion are not entirely understood. Finally, advances in stem cell research and cell therapy in combination with new generation devices may shed a different light on myocardial recovery and develop this strategy into a realistic and effective treatment option applicable to more than a very small cohort of patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lund LH, Edwards LB, Kucheryavaya AY, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:951-64. [PubMed]
- Beckmann A, Funkat AK, Lewandowski J, et al. Cardiac surgery in Germany during 2012: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2014;62:5-17. [PubMed]
- Available online: www.eurotransplant.org.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Galvao M, Saeed O, Immekus J, et al. An International Survey to Assess Referral Thresholds for Destination Therapy in Non-Inotrope Dependent Patients: Results of the CONSENSUS-DT Study. J Card Fail 2014;20:492-7. [PubMed]
- Teuteberg JJ, Stewart GC, Jessup M, et al. Implant strategies change over time and impact outcomes: insights from the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). JACC Heart Fail 2013;1:369-78. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg 2012;144:584-603. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant 2008;27:1065-72. [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803-69. [PubMed]
- Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation 2010;122:173-83. [PubMed]
- Porepa LF, Starling RC. Destination therapy with left ventricular assist devices: for whom and when? Can J Cardiol 2014;30:296-303. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826-34. [PubMed]
- Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechani-cally Assisted Circulatory Support). J Am Coll Cardiol 2014;64:1751-7. [PubMed]
- Strueber M, Larbalestier R, Jansz P, et al. Results of the post-market Registry to Evaluate the HeartWare Left Ventricular Assist System (ReVOLVE). J Heart Lung Transplant 2014;33:486-91. [PubMed]
- Beyersdorf F, Schlensak C, Berchtold-Herz M, et al. Regression of “fixed” pulmonary vascular resistance in heart transplant candidates after unloading with ventricular assist devices. J Thorac Cardiovasc Surg 2010;140:747-9. [PubMed]
- Martin J, Siegenthaler MP, Friesewinkel O, et al. Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. Eur J Cardiothorac Surg 2004;25:971-7. [PubMed]
- Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg 2007;83:1697-705. [PubMed]
- Zimpfer D, Zrunek P, Roethy W, et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg 2007;133:689-95. [PubMed]
- Atluri P, Goldstone AB, Fairman AS, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg 2013;96:857-63. [PubMed]
- Aissaoui N, Morshuis M, Schoenbrodt M, et al. Temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J Thorac Cardiovasc Surg 2013;146:186-91. [PubMed]
- Segura AM, Gregoric I, Radovancevic R, et al. Morphologic changes in the aortic wall media after support with a continuous-flow left ventricular assist device. J Heart Lung Transplant 2013;32:1096-100. [PubMed]
- Segura AM, Gregoric I, Radovancevic R, et al. Morphologic changes in the aortic wall media after support with a continuous-flow left ventricular assist device. J Heart Lung Transplant 2013;32:1096-100. [PubMed]
- Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg 2008;33:679-84. [PubMed]
- Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail 2010;3:675-81. [PubMed]
- Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg 2010;90:1263-9. [PubMed]
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207-13. [PubMed]
- Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol 2009;53:2162-7. [PubMed]
- Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2012;31:715-8. [PubMed]
- Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg 2010;25:352-6. [PubMed]
- Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol 2013;61:153-63. [PubMed]
- Boyle AJ, Jorde UP, Sun B, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880-8. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- Slaughter MS. Implantation of the HeartWare® left ventricular assist device. Semin Thorac Cardiovasc Surg 2011;23:245-7. [PubMed]
- Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2011;30:849-53. [PubMed]
- Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500-5. [PubMed]
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2011;30:164-74. [PubMed]
- Sinha P, Chen JM, Flannery M, et al. Infections during left ventricular assist device support do not affect posttransplant outcomes. Circulation 2000;102:III194-9. [PubMed]
- Kalavrouziotis D, Tong MZ, Starling RC, et al. Percutaneous lead dysfunction in the HeartMate II left ventricular assist device. Ann Thorac Surg 2014;97:1373-8. [PubMed]
- Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant 2009;28:881-7. [PubMed]
- Menon AK, Götzenich A, Sassmannshausen H, et al. Low stroke rate and few thrombo-embolic events after HeartMate II implantation under mild anticoagulation. Eur J Cardiothorac Surg 2012;42:319-23. [PubMed]
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011;92:1406-13. [PubMed]
- Kato TS, Schulze PC, Yang J, et al. P re-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant 2012;31:1-8. [PubMed]
- Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2013;96:2161-7. [PubMed]
- Nakajima I, Kato TS, Komamura K, et al. Pre- and post-operative risk factors associated with cerebrovascular accidents in patients supported by left ventricular assist device. -Single center’s experience in Japan. Circ J 2011;75:1138-46. [PubMed]
- Morgan JA, Brewer RJ, Nemeh HW, et al. Stroke while on long-term left ventricular assist device support: incidence, outcome, and predictors. ASAIO J 2014;60:284-9. [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [PubMed]
- Shah P, Mehta VM, Cowger JA, et al. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant 2014;33:102-4. [PubMed]
- Boyle AJ, Jorde UP, Sun B, et al. HeartMate II Clinical Investigators Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880-8. [PubMed]
- Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500-5. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Hoefer D, Velik-Salchner C, Antretter H. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1464. [PubMed]
- Nishimura M, Ogiwara M, Ishikawa M. Fifteen-month circulatory support for sustained ventricularfibrillation by left ventricular assist device J Thorac Cardiovasc Surg 2003;126:1190-2. [PubMed]
- Oz MC, Rose E, Slater J, et al. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J Am Coll Cardiol 1994;24:1688-91. [PubMed]
- Ziv O, Dizon J, Thosani A, et al. Effects of left ventricular assist device therapy on ventricular arrhythmias J Am Coll Cardiol 2005;45:1428-34. [PubMed]
- Cantillon DJ, Saliba WI, Wazni OM, et al. Low cardiac output associated with ventricular tachyarrhythmias in continuous-flow LVAD recipients with a concomitant ICD (LoCo VT Study). J Heart Lung Transplant 2014;33:318-20. [PubMed]
- Garan AR, Yuzefpolskaya M, Colombo PC, et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol 2013;61:2542-50. [PubMed]
- Mancini DM, Beniaminovitz A, Levin H, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation 1998;98:2383-9. [PubMed]
- Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg 1999;68:734-41. [PubMed]
- Dandel M, Weng Y, Siniawski H, et al. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 2005;112:I37-45. [PubMed]
- Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873-84. [PubMed]
- Krabatsch T, Schweiger M, Dandel M, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg 2011;91:1335-40. [PubMed]
- Devore AD, Mentz RJ, Patel CB. Medical management of patients with continuous-flow left ventricular assist devices. Curr Treat Options Cardiovasc Med 2014;16:283. [PubMed]
- Rosenbaum AN, John R, Liao KK, et al. Survival in elderly patients supported with continuous flow left ventricular assist device as bridge to transplantation or destination therapy. J Card Fail 2014;20:161-7. [PubMed]
- Oz MC, Gelijns AC, Miller L, et al. Left ventricular assist devices as permanent heart failure therapy: the price of progress. Ann Surg 2003;238:577-83. [PubMed]
- Miller LW, Nelson KE, Bostic RR, et al. Hospital costs for left ventricular assist devices for destination therapy: lower costs for implantation in the post-REMATCH era. J Heart Lung Transplant 2006;25:778-84. [PubMed]
- Clegg AJ, Scott DA, Loveman E, et al. Clinical and cost-effectiveness of left ventricular assist devices as destination therapy for people with end-stage heart failure: a systematic review and economic evaluation. Int J Technol Assess Health Care 2007;23:261-8. [PubMed]
- Slaughter MS, Bostic R, Tong K, et al. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg 2011;26:535-41. [PubMed]