Transapical aortic valve implantation - The Leipzig experience
Background: Transcatheter aortic valve implantation (TAVI) represents a significant development in the
treatment of high risk patients with aortic stenosis. As one of the first centers to perform transapical TAVI
(taTAVI), we herein review our five-year experience with this technique.
Methods: All patients undergoing taTAVI with an Edwards Sapien valve at the Leipzig Heart Center
between 2006 and 2011 (n=439) were analysed. Data was drawn from a prospective database and
retrospectively analysed. The learning curve was reviewed by means of descriptive statistics as well as
cumulative sum failure analysis (CUSUM). All results are presented in compliance with Valve Academic
Research Consortium (VARC) criteria.
Results: The mean patient age was 81.5±6.4 years and 64.0% were female. The mean logistic EuroSCORE
and STS risk of mortality were 29.7%±15.7% and 11.4%±7.6%, respectively. Procedural success was 90.2%.
Stroke occurred in 2.1% of patients intra-operatively and a further 2.1% suffered stroke during their hospital
stay. Mean transvalvular gradient was 9.0±3.9 mmHg and effective valve orifice area 1.3±0.6 cm2. Moderate
or greater aortic insufficiency was present in 5.7% of patients and remained stable during follow up. Overall
survival was 90% at 30 days, 73% at 1 year, 68% at 2 years, 58% at 3 years, 53% at 4 years, and 44% at 5 years.
CUSUM analysis revealed a definitive learning curve regarding the occurrence of major complications, with
a progressive improvement after the initial 150 cases.
Conclusions: TaTAVI has become a routine approach for high risk patients with symptomatic severe aortic
stenosis. Although taTAVI is a safe procedure with reproducible results, future research should focus on
methods of reducing known complications and the associated learning curve for this procedure.
Key words: Transcatheter aortic valve implantation (TAVI) ; transapical TAVI (taTAVI) ; aortic stenosis
Introduction
Transcatheter aortic valve implantation (TAVI) has become a dominant topic in the field of cardiac surgery and cardiology over the last few years. TAVI has enabled treatment of severe aortic stenosis in a group of patients that previously had been denied surgery because of prohibitively high operative risk (1,2). This new approach has also led to important developments such as the formation of Heart Teams at many cardiovascular centers, new research in the field of aortic root anatomy and physiology, and integration of imaging modalities into preoperative planning and procedure guidance. Moreover, a completely new clinical research field on aortic valve disease has evolved including better definition of indications for therapy, quantification of frailty as a new means for decision making, influence of different therapies on outcomes, and the impact of less commonly studied complications of aortic valve replacement therapy such as paravalvular leaks or frequent pacemaker implantation.
Our institution was one of the first to perform transapical (ta) TAVI and has thus gathered one of the largest experiences to date with this innovative and rapidly evolving technology. The vast majority of our taTAVI implantations have been performed with the Sapien valve and its various iterations (Cribier-Edwards, Edwards Sapien THV, Edwards Sapien XT; Edwards Lifesciences, Irvine, CA). The current article represents a five-year single centre experience with taTAVI using the Sapien valve, using outcome data compliant with the Valve Academic Research Consortium (VARC) guidelines.
Methods
Patient screening and selection
We performed the first two taTAVI implantations in December 2004 and then stopped the program because of paravalvular leaks requiring conventional surgery in both patients. After implementation of the oversizing concept with successful results, we restarted our taTAVI program in 2006. Since then, we have screened all patients >75 years old and with a EUROScore >9 points as possible candidates for TAVI (i.e. transapical or transfemoral). Additionally, patients with uncommon but significant risk factors for conventional surgery were considered for a TAVI procedure. Such risk factors included porcelain aorta, previous chest radiation, previous mediastinitis, status post coronary bypass grafting with patent grafts, severe COPD, and cirrhosis. The final decision for or against TAVI, as well as which transcatheter approach to employ, was made on an individual basis by a team of at least one cardiac surgeon and one cardiologist.
Patients who were possible candidates for TAVI went through a standard preoperative diagnostic workup including transthoracic echocardiography, pulmonary function tests, EKG, CXR, and baseline bloodwork. Additionally, a transoesophageal echo was performed in all patients to determine the exact annulus size. With the availability of multislice computer tomography (MCT) and specialized software programs, preoperative CT scanning became more predominant over time as the method of choice for annulus measurement.
Surgical technique
All taTAVI implantations were performed in a hybrid operation suite by a team of at least one cardiac surgeon, one cardiologist, and one anaesthetist. Preparation and crimping of the valve prosthesis was done by a trained perfusionist, who was also responsible for emergency institution of cardiopulmonary bypass (CPB) when required.
The technique for taTAVI has been described elsewhere and has basically remained unchanged throughout the years (3). In short, a long wire is advanced into the right atrium through a femoral vein puncture and a 6F sheath is inserted into the femoral artery, through which a pigtail catheter for aortic root angiography is inserted. Both percutaneous access sites act as a “safety net” and can be rapidly exchanged for CPB cannulas if necessary.
A left-sided minithoracotomy is performed in the 5th or 6th intercostal space in order to visualize the apex of the heart. Pericardial stay sutures are placed in order to stabilize the operative field. Two pledgeted 2-0 Prolene purse-string sutures (or alternatively, two U-stitches) are placed slightly anterior and lateral to the anatomical apex, ensuring that a muscular region of the left ventricle is incorporated. The procedure is thereafter performed under fluoroscopic guidance, using high intensity imaging.
It is essential during fluoroscopic positioning to find a perpendicular view of the aortic annulus. At the beginning of our TAVI experience, the optimal fluoroscopic angulation was found by performing multiple contrast aortic root injections. With the development of DynaCT software (Siemens; Erlangen, Germany), a threedimensional reconstruction of the aortic root can now be achieved by performing a rotational angiography during rapid ventricular pacing, immediately following injection of 15 mL of contrast medium through the pigtail catheter. The optimal angulation can thus be defined with the use of very little contrast medium. An even better alternative is to import a preoperatively performed CT into the imaging system and have it analysed by the software. This method has the advantage of not only reducing contrast medium administration, but also reduces the amount of radiation exposure.
The left ventricle is punctured through the previously placed purse-string sutures and a soft J-wire is advanced into the ascending aorta under fluoroscopic guidance. A 14F soft tip sheath is then advanced over the J-wire and through the aortic valve. A super stiff guidewire is then placed in the descending aorta (with the help of a right Judkins catheter), and a balloon valvuloplasty is performed in 100% of cases. The 14F soft tip sheath is than exchanged for the particular implant device. Implantation of a self-expanding prosthesis is most often done without rapid pacing. For the balloonexpandable Sapien valve, however, implantation is always performed during rapid ventricular pacing. A final shot of contrast is administered into the aortic root during slow deflation of the balloon, enabling minor corrections of valve positioning during the actual implantation process.
Proper valve function is always confirmed by both TEE and root angiography before proceeding with wound closure. If the patient develops any signs of hemodynamic instability, possible causes are immediately investigated by TEE and coronary angiography.
Data analysis
The data of all patients who underwent a taTAVI procedure with the Sapien valve from 2006 to 2011 were retrospectively analysed. The data was drawn from a prospective database that was developed at the very beginning of our TAVI program. The results were analysed in detail according to the VARC definitions and criteria (4). Since the transapical program at the Leipzig Heart Center was one of the first worldwide, we also analysed our learning curve by calculation of descriptive statistics and cumulative sum (CUSUM) failure analysis, which is described in detail elsewhere (5).
ategorical variables are expressed as proportions and continuous variables as mean +/- standard deviations throughout the manuscript.
Results
TAVI case number development
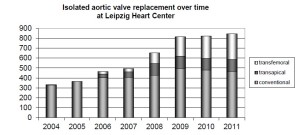
Since the beginning of our TAVI program (transfemoral and transapical) in 2006, the annual number of procedures performed has continuously risen and reached more than 350 in 2011. It is interesting to note that during the same 5-year time period the number of conventional aortic valve replacement operations did not decrease, but rather increased slightly (Figure 1). The rapid growth in total aortic valve procedures that we have observed over the last 5 years has been most probably due to a combination of factors including our early adoption of TAVI with early publication of our results, the increased referral of high risk patients who may have been previously thought to be unsuitable for conventional surgery, and the natural increase in the number of patients presenting with aortic stenosis as the average age of the general population increases. In the beginning of our TAVI program, the transapical approach was dominant because of our principal role in developing this technique. With increasing experience with transfemoral TAVI and the development of more refined and smaller gauge transfemoral devices, however, the transapical to transfemoral ratio has steadily decreased and is now approximately 1:2 (Figure 1).
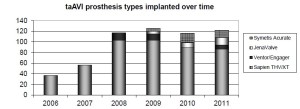
We have gained experience with several different taTAVI prosthesis types over the last five years (Figure 2). The following data refers only to our taAVI experience with the various iterations of the Edwards balloon expandable valve (Cribier Edwards n=134, Sapien THV n=189, and Sapien XT n=222). Refinements that have we have observed in the Sapien valve system over time include the switch from equine to bovine pericardium, use of anticalcification treatment, an increased number of available valve sizes, and smaller and more flexible delivery systems that are better able to be handled by one operator.
Patient characteristics and short term outcomes
The mean age of the first 439 consecutive taTAVI Sapien patients was 81.5±6.4 years and 281 patients (64.0%) were female. The mean Logistic EuroSCORE and Society of Thoracic Surgeons Score predicted risks for mortality were 29.7±15.7% and 11.4±7.6%, respectively. Preoperative New York Heart Association functional status was II in 65 (14.8%), III in 290 (66.1%), and IV in 81 (18.5%) patients. Additional data on preoperative patient characteristics are supplied in Table 1.
| Table 1 Preoperative patient characteristics | |
| Patients | n=439 |
|---|---|
| Age, y | 81.5±6.4 (73.2-88.3) |
| Female, % | 281 (64.0%) |
| Body height, cm | 162.0 (152.0-175.0) |
| Weight, kg | 68.0 (52.0-90.0) |
| NYHA class | 3.0 (2.0-4.0) |
| Cardiac redo procedure (not valvular), % | 125/29.4 |
| Left ventricular ejection fraction, % | 54.7±13.4 |
| Peripheral vascular disease, % | 79/18.0 |
| Coronary artery disease, % | 56.8 |
| Chronic obstructive lung disease, % | 36.1 |
| Pulmonary hypertension > |
28.7 |
| Diabetes, % | 43.4 |
| Chronic renal insufficiency (creatinine>2 mg/dL), % | 10.4 |
| Permanent atrial fibrillation, % | 8.6 |
| FEV1, % of normal | 89.0 (54.6-127.0) |
| Additive EuroSCORE | 11.5±2.3 |
| logistic EuroSCORE, % | 29.7±15.7 |
| STS Score | 11.4±7.6 |
| Continuous variables expressed as mean +/- standard deviation or mean (interquartile range). FEV1, forced expiratory volume in one second; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons | |
Intra-procedural device success as defined by VARC was 90.2%. CPB was used by intention in 10 patients at the beginning of our experience. Thereafter, a total of 17 (4.0%) of the remaining 429 patients had to be converted to CPB due to hemodynamic instability, coronary ischemia, annular tear, valve dysfunction requiring valve-in-valve implantation, apical bleeding, or conversion to conventional AVR surgery (2.5%).
The procedure was uneventful in 406 patients, whereas 33 patients (7.5%) required additional interventions: (I) Coronary intervention in 6 patients (1.4%) owing to coronary occlusion by the prosthesis (n=2), embolization of calcium (n=1), injury of a venous bypass graft during sternotomy due to valve embolization (n=1), and stenosis of the LIMA graft in patients who underwent concomitant elective minimally invasive direct coronary artery bypass surgery (n=2). (II) Requirement for the implantation of a second SAPIEN valve in 24 (5.5%) patients owing to a valve position that was too high (n=10) or too low (n=2), leaflet dysfunction in the first and second generation valves (n=8), ventricular septal defect (n=3), or upside down valve positioning (n=1). (III) Annulus perforation requiring conventional surgery in 3 patients (0.6%). For more intraoperative details see also Table 2.
| Table 2 TransapicalSapien implantation data | |
| All patients | n=439 |
|---|---|
| Preop mean aortic gradient, mmHg | 45.7±18.0 |
| Preop maximal aortic gradient, mmHg | 71.8±25.2 |
| Preop aortic valve orifice area, cm2 | 0.58±0.20 |
| TEE annulus diameter, mm | 23.0 (20.0-25.0) |
| Off-pump procedure, n (%) | 412 (93.8) |
| Conversion to sternotomy and AVR, n (%) | 11 (2.5) |
| Conversion to CPB, n (%) | 27 (6.2) |
| Valve implantation, SapienEdwards* |
138 (31.4) |
| Re-balloning during index procedure, n (%) | 38 (8.7) |
| Additional apical suturing, n (%) | 43 (9.8) |
| Contrast dye application**, mL | 90.0 (60.0-145.0) |
| X-ray time, min | 5.5 (3.3-10.0) |
| Procedural time, min | 75.0 (56.8-135.5) |
| Continuous variables expressed as mean +/- standard deviation or mean (interquartile range). *By intention-to-treat, ** type of contrast media; Ultravist 370 TEE, transesophageal echocardiography; AVR = aortic valve replacement surgery; CPB, cardiopulmonary byass | |
Peri-procedural stroke was observed in 2.1% of patients and postoperative stroke was observed in a further 2.1% of patients. Of the 19 patients with neurologic complications, 1 experienced a transient ischemic attack, 7 experienced a minor stroke and 11 a major stroke.
Other peri-procedural complications according to VARC criteria consisted of major vascular complications in 3.4% of patients, life-threatening or disabling bleeding in 6.2%, and acute kidney injury in 27.9% of patients (modified RIFLE classification stage 1: 8.3%, stage 2: 3.3% and stage 3: 16.3%).
Early postoperative prosthetic valve performance was good with maximal and mean gradients of 16.3±6.5 and 9.0±3.9 mmHg, respectively. Effective valve orifice area was 1.33±0.61 cm2 at discharge. None or trivial aortic insufficiency (AI) was present in 60.6% of patients, mild AI in 33.7%, moderate AI in 5.2%, and severe AI in 0.5%. One patient (0.2%) developed endocarditis early postoperatively, while there was no patient with prosthetic valve thrombosis.
New onset of atrial fibrillation was observed in 27.2% of patients, whereas new left bundle branch block and third degree atrioventricular block was found in 27 (6.2%) and 5 (1.1%) patients, respectively within 30 days of the index procedure. A new pacemaker was required in 11.2% of patients.
Survival and long term outcomes
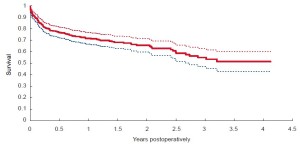
Overall survival was 90% at 30 days, 73% at 1 year, 68% at 2 years, 58% at 3 years, 53% at 4 years, and 44% at 5 years for the entire group of Sapien patients (Figure 3). Total mortality during the follow-up interval of 2051 days (interquartile-range, 159 to 1,050 days) was 36.2%.
Long-term echocardiographic follow up, although available in a relatively small number of patients, revealed stable hemodynamic performance over time (Table 3). In particular, gradients remained low and no obvious increase in paravalvular leaks was observed.
| Table 3 Echocardiographic results over time | ||||||
| N | Discharge |
One year |
Two years |
Three years |
Four years |
Five years |
|---|---|---|---|---|---|---|
| Vmax (m/s) | 2.4 | 2.1 | 2.0 | 2.1 | 2.1 | 2.2 |
| Pmax (mm Hg) | 15.4 | 17.7 | 16.3 | 17.9 | 18.2 | 19.6 |
| Pmean (mm Hg) | 8.2 | 9.7 | 8.8 | 9.5 | 9.5 | 11.5 |
| LVEF (%) | 54.9 | 58.1 | 57.6 | 59.3 | 58.2 | 62.7 |
| AI | ||||||
| Mild (first degree) | 34.3% | 40.8% | 42.4% | 51.6% | 40.0% | 33.3% |
| Moderate | 5.4% | 3.5% | 4.6% | 6.5% | 6.7% | 33.3% |
| Vmax: Maximum velocity; Pmax: Maximum pressure gradient; Pmean: Mean pressure gradient; LVEF: Left ventricular ejection fraction; AI: Aortic insufficiency | ||||||
Learning curve
We also analysed the learning process associated with taTAVI at our centre. Our learning curve was particularly of interest given that we did not have the experience of other centres to learn from. It included all aspects of the operation including patient selection and indications, apical access, adoption of wire skills and fluoroscopic imaging, switching from an on-pump to off-pump approach, installation of a “safety net”, postoperative care including initially unexpected complications (e.g., late AV block), and, last but not least, the individual surgeon’s learning curve that can be expected with any new procedure. The number of surgeons involved in the transapical program so far has been kept to a small number (n=4) in order to keep the number of cases per surgeon high.
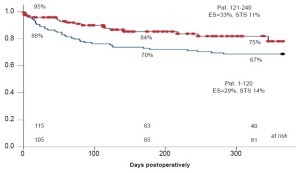
Figure 4 compares the Kaplan-Meier survival curves of the first 120 consecutive taTAVI patients with the second 120 taTAVI patients. Although patients had an almost identical risk profile, the one-year survival was markedly better in the latter group. We believe that improvements in postoperative monitoring and complication management lead to a particular improvement in outcomes.
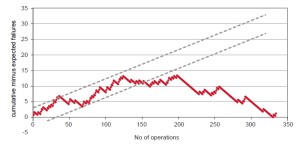
The overcoming of the learning curve was also confirmed by a cumulative failure sum analysis. Figure 5 shows the result of a sequential probability CUSUM analysis of all major complications including conversion to sternotomy, stroke, new dialysis, low cardiac output with need for ECMO or IABP, reoperation for bleeding or valve dysfunction, long term dependency on respirator/tracheotomy, and death. The curve was calculated with an expected rate of occurrence of any of these complications of 15%. As can be seen, the graph levels off after approximately 120 procedures and a statistically significant improvement in performance occurs after 150 procedures. After a total of 200 taTAVI procedures have been performed, the curve is progressively downward indicating that the learning phase has been overcome and that steady improvement is occurring.
Discussion
The present series represents one of the largest singlecentre experiences for taTAVI worldwide and includes the initial learning curve (i.e. early 2006), when very few centres were performing these procedures. The results are generally very good and comparable to those reported in other series.
The incidence of peri-procedural stroke was 2.1% in our series and a further 2.1% of patients experienced a stroke some time during their hospital stay. The proportion of patients experiencing any major stroke (peri-procedural or during hospital stay) is lower than that observed in the transfemoral group of the Partner Trial, cohort B (2.5% vs. 5.0%), most likely due to decreased manipulations of the aortic arch when using the antegrade transapical approach (1). Similarly, several reports on TAVI in the literature have displayed a very low or absent stroke rate when using an antegrade transapical approach (6-9).
Transcatheter-related bleeding complications have been reported in up to 31% of TAVI patients (10). The presented study showed an incidence of life-threatening bleeding in 6.2%, which is similar to the results of 2 recent studies using the transfemoral approach (10,11). In the Partner B trial (transfermoral n=179), the rate of major bleeding complications was 16.8% using the modified VARC definition, while 9.3% of patients in Partner A (transfemoral n=244, transapical n=104) developed this complication. It should be noted, however, that some patients developed vascular complications without bleeding, while some had life-threatening gastro-intestinal bleeds. We also observed a significant proportion of patients undergoing blood transfusions, and therefore classified as severe bleeding according to the VARC criteria, without an obvious source of bleeding. Whether the need for transfusion was related to pre-existing anaemia or preoperative cardiac catheterization is unclear, but the rate of major bleeding may be overestimated using the VARC criteria. Future revisions of these criteria may need to take this limitation into account.
Acute kidney injury (AKI) is one of the most serious complications following TAVI due to its strong impact on short- and long-term mortality (12). Our observed frequency of stage 3 AKI of 16.3% is similar to those from previous studies reporting a frequency ranging from 12% to 28% (13,14). We have implemented several alterations to our taAVI procedure in order to minimize the amount of contrast medium administered and try to minimize the risk of severe AKI occurring (12).
Our observed rate of new permanent pacemaker insertion of 11.2% was a little higher than that in previously published series using the Edwards SAPIEN prosthesis (15-17). The increased pacemaker rate is partly due to a liberalization of our indications for pacemaker implantation, reflecting our observation early in the series that late AV block occasionally occurs several days post-TAVI. Because of these rare but life-threatening experiences, we currently perform holter monitoring for all TAVI patients up to 5 days post-procedure. Our relatively high pacemaker implantation rate continues to be much lower than those reported for the CoreValve prosthesis (18).
In the current series, annular perforation was a rare but life-threatening complication that occurred in 3 patients (0.6%). On the basis of this limited experience, identification of specific risk factors for this complication has been difficult.
Our echocardiographic results and those from other centres reveal excellent early hemodynamic performance for transcatheter valves, rivalling those of stentless aortic bioprostheses. However, long-term results are not yet known and will be critical for the future decision of whether or not to perform TAVI in younger patients. In addition, the significant problem of paravalvular leakage is an issue that all transcatheter valve companies will need to further address. We observed a paravalvular leak rate of almost 40% in our study, although it was mild in the vast majority of patients and only 2 required reoperation for increasing aortic incompetence.
The 30-day mortality of our entire patient population was 9.6%. The rate observed in our series was similar to that reported in previous registries: Canadian registry, 10.4% (19); SOURCE (SAPIEN Aortic Bioprosthesis European Outcome) registry, 8.5% (20); FRANCE (French Aortic National CoreValve and Edwards) registry, 12.7% (21); German registry, 8.2% (22); and Italian registry, 5.4% (23). In the PARTNER Trial (cohort B) 30-day mortality was 5%, and it was 5.2% in the PARTNER A cohort (1,2).
Future areas of research will need to focus on lowering the rates of the abovementioned complications associated with TAVI. In addition, attempts should be made to shorten the observed taTAVI learning curve for those centres that are just now embarking on these programs. Possible methods include the open sharing of knowledge and experience (both good and bad) between centres, the use of simulation models prior to clinical use, and the implementation of proctoring during the early phase of program development.
Limitations
The analysis is retrospective in nature with all the inherent weaknesses of a retrospective study. Long term echocardiographic and clinical follow up data is currently sparse, but efforts are ongoing in order to obtain more information on these patients.
Conclusions
Our experience shows that taTAVI with the balloonexpandable Sapien valve has become a routine procedure with good results in high risk patients with symptomatic aortic stenosis. Five-year follow up suggests continued good clinical and echocardiographic results for these high risk patients, but further data is required.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Walther T, Dewey T, Borger MA, et al. Transapical aortic valve implantation: step by step. Ann Thorac Surg 2009;87:276-83.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17.
- Holzhey DM, Jacobs S, Walther T, et al. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg 2007;134:663-9.
- Bleiziffer S, Ruge H, Mazzitelli D, et al. Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. J Thorac Cardiovasc Surg 2009;138:1073-80.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006;114:591-6.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation--proof of concept. Eur J Cardiothorac Surg 2007;31:9-15.
- Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5.
- Nuis RJ, Piazza N, Van Mieghem NM, et al. In-hospital complications after transcatheter aortic valve implantation revisited according to the valve academic research consortium definitions. Catheter Cardiovasc Interv 2011;78:457-67.
- Gurvitch R, Toggweiler S, Willson AB, et al. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention 2011;7:41-8.
- Borger MA, Holzhey DM, Mohr FW. Minimizing contrast medium dose during transapical aortic valve implantation: it is worth the effort. Eur J Cardiothorac Surg 2012;41:1232-3.
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74.
- Aregger F, Wenaweser P, Hellige GJ, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant 2009;24:2175-9.
- Moat NE, Ludman P, Belder MA, et al. Long-Term Outcomes After Transcatheter Aortic Valve Implantation in High-Risk Patients With Severe Aortic Stenosis The U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8.
- Thomas M, Schymik G, Walther T, et al. Oneyear outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011;124:425-33.
- Wendler O, Walther T, Nataf P, et al. Trans-apical aortic valve implantation: univariate and multivariate analyses of the early results from the SOURCE registry. Eur J Cardiothorac Surg 2010;38:119-27.
- Grube E, Buellesfeld L, Mueller R, et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circ Cardiovasc Interv 2008;1:167-75.
- Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90.
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62-9.
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7.
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre realworld registry. Eur Heart J 2011;32:198-204.
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308.