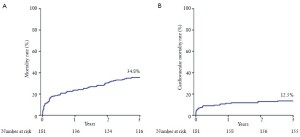
3-year outcomes of self-expanding Corevalve prosthesis - The Italian Registry
Transcatheter aortic valve implantation (TAVI) is an emerging, catheter-based technology that allows for implantation of a prosthetic valve without open heart surgery for the treatment of severe aortic stenosis (AS) (1). The recently published Placement of Aortic Trans Catheter Valves (PARTNER) randomized controlled trial cohort B demonstrated that TAVI remarkably reduced the mortality, as compared with standard therapy, in patients deemed unsuitable for surgery (2). In addition, PARTNER cohort A results showed that 1-year outcomes after TAVI compare favorably with surgical aortic valve replacement (3). While a number of large single-arm registries demonstrated encouraging short and mid-term effectiveness of TAVI (4-9), there is a paucity of data on the benefits of this technique at longer follow-up (10-11). The lack of evidence for the longterm durability of currently available transcatheter heart valves is one of the main issues preventing TAVI being used in younger and lower risk patients. The present study evaluated the medium- to long-term outcomes of an early cohort undergoing transcatheter aortic valve implantation with the third generation (18-Fr) CoreValve prosthesis (CRS) (Medtronic Incorporation, MN, USA) at 12 centers across Italy, with all patients evaluated by follow-up at a minimum of 3 years from the procedure. Device success, cardiovascular death, peri-procedural and spontaneous myocardial infarction, strokes, bleeding, combined safety and efficacy endpoints and echocardiographic criteria post- TAVI were defined according to the Valve Academic Research Consortium (VARC) (12). The mean age of the entire population was 80.9±6.1 years. All patients had severe symptomatic AS [mean aortic valve area (AVA) 0.61±0.23 cm2]. Overall, the population was at high surgical risk with a predicted 30-day mortality of 24.0±13.5% by Logistic EuroScore and 11.4±9.9% by STS-mortality score. Trans-femoral access was used in 172 patients (95.0%); in 9 patients (5.0%) where the trans-femoral approach was not feasible, a trans-subclavian approach was employed. Clinical follow-up was available in 178 patients (98.3%) at a mean of 41±3 months (range 36 to 51 months) after TAVI. All-cause mortality rates at 1, 2, and 3 years were 23.6%, 30.3%, and 34.8%, respectively (Figure 1A). Cardiovascular mortality rates at 1, 2, and 3 years were 11.2%, 12.1%, and 13.5%, respectively (Figure 1B). The bulk of late mortality in this high-risk cohort was due to significant comorbidities and was generally unrelated to aortic valve disease. The actuarial rate of a composite of death, major stroke, myocardial infarction and life-threatening bleeding was 30.1% at 1 year, 36.5% at 2 years, and 40.3% at 3 years. Patients with renal insufficiency did not present significant differences in term of 30-day mortality (3.8% vs. 7.8%; P=0.332) compared with those without, whereas they had higher mortality at 3-year follow-up (51.0% vs. 29.2%, P=0.007); on the other hand, patients experiencing post-procedural major or lifethreatening bleeding had a higher rate of mortality already at 30 days (21.6% vs. 2.8%; P<0.001) and this result was sustained at 3-year follow-up (62.2 % vs. 28.4%; P<0.001). Moreover, the study demonstrated excellent durability, no evidence of structural valvular failure, and preserved hemodynamics. No changes in valve area and transvalvular gradients were documented, which were generally different to those in previously published surgical series that reported on bioprosthetic valves in the aortic position. Patients showed significant improvement in functional state, which appeared to be preserved over time. Postprocedural aortic regurgitation was generally mild and did not appear to worsen over time. Echocardiographic study at followup also demonstrated no evidence of valve fracture, deformation, or valve migration.
The central finding of this study is that 3-year survival after TAVI with the CRS prosthesis amounted to 65%. This data is not surprising, given the baseline risk profile of the subjects undergoing the procedure during the early TAVI experience which was extremely high. Therefore, patients died during follow-up either because of their comorbidities or secondary to conditions associated with advanced age, as proved by the 13% of cardiovascular death rate at 3 years, which mostly occurred during the first month after TAVI, due to procedural complications. This poses an obvious challenge for longer-term real effectiveness of TAVI in such a population and raises questions about whether this procedure could improve long-term results in lower-risk patients, as actually suggested by recent reports (9,13). According to our findings, since three out of five patients died due to non-cardiovascular causes at a median of 11 months, almost half of those patients should be denied the procedure in agreement of 2008 European position statement on TAVI, which pointed out that this procedure should not be performed in patients whose life expectancy is less than 1 year (14). Therefore, in the future it will be crucial to identify patient populations that can derive most advantage from the application of this technology in the perspective of a cost-benefit ratio. Overall, when used in patients who are deemed to be poor surgical candidates, transcatheter aortic valve implantation appears to offer an adequate and lasting resolution of symptomatic aortic stenosis.
Acknowledgements
Disclosure: Gian Paolo Ussia, is a proctor physician for Medtronic Incorporation; all other authors have no relevant conflicts of interests to declare.
References
- Webb J, Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J 2011;32:140-7.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic valve implantation in inoperable patients with severe aortic stenosis. N Engl J Med 2010;363:1597-607.
- Smith CR, Leon MB, Mack M, et al. for the PARTNER Trial Investigators. Transcatheter versus Surgical Aortic- Valve Replacement in High-Risk Patients. N Engl J Med 2011;364:2187-98.
- Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter Aortic Valve Implantation for the Treatment of Severe Symptomatic Aortic Stenosis in Patients at Very High or Prohibitive Surgical Risk Acute and Late Outcomes of the Multicenter Canadian Experience. J Am Coll Cardiol 2010;55:1080-90.
- Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J 2011;32:191-7.
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre realworld registry. Eur Heart J 2011;32:198-204.
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and Predictors of Early and Late Mortality after Transcatheter Aortic Valve Implantation in 663 Patients with Severe Aortic Stenosis. Circulation 2011;123:299-308.
- Kodali SK, O’Neill WW, Moses JW, et al. Early and late (one year) outcomes following transcatheter aortic valve implantation in patients with severe aortic stenosis (from the United States REVIVAL trial). Am J Cardiol 2011;107:1058-64.
- Thomas M, Schymik G, Walther T, et al. One-Year Outcomes of Cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry: The European Registry of Transcatheter Aortic Valve Implantation Using the Edwards SAPIEN Valve. Circulation 2011;124:425-33.
- Gurvitch R, Wood DA, Tay EL, et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 2010;122:1319-27.
- Buellesfeld L, Gerckens U, Schuler G, et al. 2-year followup of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol 2011;57:1650-7.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials a consensus report from the valve academic research consortium. Eur Heart J 2011;32:205-17.
- Tamburino C, Barbanti M, Capodanno D, et al. Early and mid term outcomes of transcatheter aortic valve implantation in patients with logistic euroscore less than 20%: A comparative analysis between different risk strata. Catheter Cardiovasc Interv 2012;79:132-40.
- Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2008;29:1463-70.