The role of adjuvant chemotherapy following resection of early stage thymoma
Introduction
Thymoma is the most common thymic epithelial tumor (TET). Completeness of resection has consistently been shown to be a critical factor for determining recurrence and overall survival in early stage thymoma (1-4).
Adjuvant chemotherapy appears to be less frequently administered than adjuvant radiotherapy and is mostly reserved for advanced stage TETs (5,6). There is a paucity of published data regarding adjuvant chemotherapy following resection of early stage thymomas and there remains no standard guideline for its management. Given the absence of high quality evidence and the small sizes of relevant published cohorts, a systematic review was performed to characterize outcomes in patients undergoing adjuvant chemotherapy following surgical resection of early stage thymoma.
Materials and methods
PubMed (United States National Library of Medicine) database was searched from its date of inception to the 26th June 2015 for English-language studies, using the terms “thymoma” AND “adjuvant” AND chemotherapy “AND “surgery”. The study selection process is summarized in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart (Figure 1). Final eligibility criteria were original studies reporting outcomes of patients undergoing adjuvant chemotherapy following thymectomy for early-stage (Masaoka stage I or II) thymoma.
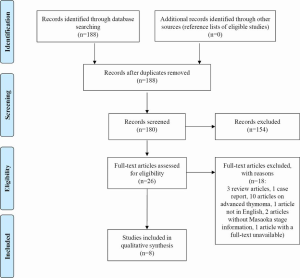
Extracted data from eligible studies included details of study design, eligibility criteria, patient characteristics, Masaoka stages, World Health Organization (WHO) histology, chemotherapy regimens, adjuvant radiotherapy, and survival outcomes.
Results
The database search yielded 188 records, of which 180 abstracts were accessible. After screening based on title and abstract content, 26 records were selected for full-text review. Finally, eight original studies reporting on Masaoka stage I or II thymoma patients undergoing adjuvant chemotherapy were eligible for inclusion (7-14). Each study met the majority of criteria according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement. A summary of study characteristics is shown in Table 1. Three studies did not provide the number of patients with stage I or II thymoma (9-11). In the remaining five studies, there were a total of 890 patients with stage I or II thymomas, of which 140 (15.7%) underwent adjuvant chemotherapy with or without radiotherapy (7,8,12-14).

Full table
Kondo et al. analyzed a Japanese multi-institutional database of 1,320 patients with TETs (8). All 522 Masaoka stage I thymomas were completely resected and, of note, 14.3% of the 522 patients underwent adjuvant chemotherapy and 3.6% underwent adjuvant chemoradiotherapy. All 247 Masaoka stage II thymomas were also completely resected and 6.5% of the 247 patients underwent adjuvant chemotherapy and 5.6% underwent adjuvant chemoradiotherapy. Information on short-term outcomes such as toxicity or long-term survival outcomes was not available for stage I or II patients undergoing adjuvant chemotherapy or chemoradiotherapy following surgery.
Kim et al. from South Korea, published their institutional experience over 10 years (10). In total, 100 patients were analyzed, with 58 patients staged as Masaoka stage II (and no patient as stage I). Of the stage II patients, 35 patients underwent adjuvant radiotherapy only and 23 patients underwent adjuvant chemoradiotherapy. The WHO histology ranged from type A to type C in stage II patients. The chemotherapy regimen was limited to six cycles of doxorubicin, cisplatin, vincristine, and cyclophosphamide (ADOC). In adjuvant chemoradiotherapy, three cycles of chemotherapy were administered first, followed by radiotherapy, and an additional three cycles of chemotherapy. No short-term mortality was noted, but long-term survival outcomes were not reported.
Froudarakis and colleagues reported on their clinical experience of 23 patients with invasive thymoma (Masaoka stage II or higher). They reported patient demographics, tumor characteristics, and treatment outcomes for all patients. Of the 23 patients, there were three stage II patients (and no stage I). Two patients underwent adjuvant chemoradiotherapy following complete resection of epithelial and mixed thymoma, respectively. Both patients were alive at latest follow-up of 155 months and 65 months, respectively. The other stage II patient underwent adjuvant radiotherapy following radiotherapy and was alive at latest follow-up of 160 months.
Ansari and colleagues reported prognostic factors for survival in 45 patients in Iran with TETs (7). Twelve patients underwent complete resection only for Masaoka stage I, with WHO histology ranging from type A to type C. Of eight stage II patients, seven patients underwent R0 or R1 resection, followed by adjuvant radiotherapy and one patient received adjuvant chemoradiotherapy following resection (whether it was R0 or R1 was not indicated in the study). The chemotherapy regimen in this study consisted of two to six cycles (median: four) of CAP (cyclophosphamide, doxorubicin, and cisplatin) with or without prednisolone. Further information on adjuvant chemotherapy was not available.
Kumar reviewed 36 patients with thymoma (9). Five patients received adjuvant chemotherapy. The Masaoka stage or resection status was unknown in the five patients. The adjuvant chemotherapy regime consisted of a median of six cycles of either cyclophosphamide, adriamycin, and cisplatin (CAP) or cisplatin and etoposide (PE). The toxicities of chemotherapy were limited to grade 1-2 hematological toxicity. Further information on adjuvant chemotherapy was not available.
Zhu and colleagues analyzed 175 patients in an attempt to investigate strategies for optimizing adjuvant radiotherapy following surgery for thymoma (12). There were a total of 175 patients, including 47 patients with non-invasive (Masaoka stage I) thymoma and 128 patients with invasive (Masaoka stage II-IV) thymoma. The indication for adjuvant chemotherapy was unresectable or incompletely resected thymoma. The chemotherapy regimen consisted of cisplatin, adriamycin, and cyclophosphamide. No patient with stage I thymoma underwent adjuvant chemotherapy, while 25 patients (19.5%) with invasive (stage II-IV) thymoma underwent adjuvant chemotherapy with radiotherapy. Survival data was not reported according to adjuvant therapy.
Strobel and colleagues aimed to investigate the impact of WHO histological stage on survival outcomes (11). The heterogeneity of adjuvant chemotherapy regimens precluded comparative analyses, and no apparent influence was found on survival outcomes of patients with R0 resected WHO B2 or B3 stage II thymomas. Details regarding adjuvant therapy were reported according to WHO histological stage rather than Masaoka stage. Adjuvant chemotherapy following surgery was administered in 1/20 (5%) of WHO type A, 1/49 (2.0%) of type AB, 0% of type B1, 1/40 (2.5%) of type B2, and 4/22 (18.2%) of type B3 thymomas. Adjuvant chemoradiotherapy following surgery was performed in 1/20 (5%) of type A, 0% of type AB or B1, 4/40 (10%) of type B2, and 4/22 (18.2%) of type B3 thymomas. There was no information available on WHO histologies of patients with early stage thymoma.
Venuta and colleagues performed a prospective observational study on multimodality treatments for thymoma (14). They grouped their patients on the basis of histology combined with Masaoka histology: medullary stage II and medullary or mixed-type stage I (group I), cortical stage I and II or mixed-type stage III (group II), and cortical stage III and IV or mixed-type stage III (group III). No adjuvant therapy was given to group I patients, adjuvant chemoradiotherapy was given to group II patients, and neoadjuvant chemotherapy and adjuvant chemoradiotherapy were given to group III patients. Group II patients included 11 stage I and 1 stage II patients. No stage I or II patient was included in group III. Overall survival of group II patients showed an improved (but not significant) trend, compared to a historical control.
Discussion
The goal of surgery for early stage thymoma is complete resection (1), which is a significant favorable prognostic factor for overall survival (2-4). Late recurrence following resection is not uncommon (15) and adjuvant therapy following initial resection may reduce the rate of recurrence.
Ruffini and colleagues analyzed the largest cohort (the European Society of Thoracic Surgeons database) of patients with TETs (including thymoma, thymic carcinoma, and thymic carcinoid) and concluded that adjuvant therapy (including radiotherapy and chemotherapy) following complete resection is associated with improved overall survival. However, the analyses were not specific to adjuvant chemotherapy for R0 resected thymomas (16). In a subsequent report, Ruffini et al. provided futher details on adjuvant therapy following complete resection for thymoma patients. Amongst 1,519 patients undergoing complete resection of thymoma, 28 patients (1.8%) received adjuvant chemotherapy only, 118 (7.8%) received adjuvant chemoradiotherapy, and 436 (28.7%) received adjuvant radiotherapy only. Results indicated that adjuvant chemotherapy alone following complete resection did not result in a significant advantage, while adjuvant radiotherapy or chemoradiotherapy did (17). Analyses according to Masaoka stage were beyond the scope of the study and discussions.
According to NCCN guidelines, adjuvant radiotherapy is recommended for completely resected Masaoka stage II thymoma and an incompletely resected thymoma at any stage. Limited to stage I and stage II thymomas, which presumably were completely resected, adjuvant radiotherapy might not be associated with improved overall survival (18,19).
There is a paucity of literature regarding adjuvant chemotherapy. This systematic review included eight studies published from 1997 to 2014. All included studies were retrospective reports. In total, this review included more than 140 patients who underwent adjuvant chemotherapy with or without radiotherapy following surgical resection early stage thymoma (stage I-II) in France, Italy, Germany, South Korea, China, India, Japan, and Iran (7-14).
The main information obtained from this review was the regimen, number of cycles and proportion of patients who underwent adjuvant chemotherapy or chemoradiotherapy for early-stage thymoma. First-line chemotherapy regimens recommended by NCCN guidelines were CAP (cisplatin, doxorubicin, and cyclophosphamide) with or without prednisone, ADOC (cisplatin, doxorubicin, vincristine, cyclophosphamide), PE (cisplatin and etoposide) and VIP (etoposide, ifosphamide, and cisplatin).
Except for the study of Kondo and colleagues (8), adjuvant chemotherapy was administered along with adjuvant radiotherapy (7,9-14). Adjuvant chemotherapy appeared to be ancillary to radiotherapy and was rarely administered without radiotherapy. Information on the indications for each approach was not available in any study.
Ansari, Kim, and Strobel described WHO histologies in their studies (7,10,11). Specific information on WHO histologies of patients with early stage thymoma undergoing adjuvant chemotherapy or chemoradiotherapy was not available, but WHO histologies could be a potential indication for adjuvant therapy after complete resection since WHO histology may be a significant prognostic factor for survival after resection of thymoma (20,21).
In summary, this systematic review suggested that adjuvant chemotherapy is not routinely administered in early stage thymomas with R0 resection but is in conjunction with radiotherapy. The evidence for superiority over no adjuvant chemotherapy is not available. This review was limited by a single database search, the small sample sizes of the included studies, and the completeness of reporting in included studies. No randomized controlled studies or matched cohort studies were available. None of the included studies provided statistical comparison with a concurrent control group. Given insufficient data on long-term survival outcomes, there is no evidence to recommend adjuvant chemotherapy following resection of early-stage thymomas. The developing databases of TETs such as International Thymic Malignancy Interest Group, the European Society of Thoracic Surgeons database, or Japanese Association for Research on the Thymus database, may be expected to provide rigorous survival data on this specific question.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- NCCN clinical practice guidelines in Oncology. Thymomas and Thymic Carcinomas Version 2. 2013 Available online: http://www.thymic.org/wp-content/uploads/2009/03/nccn-guidelines.pdf. Accessed May 5, 2015.
- Demirci S, Turhan K, Ozsan N, et al. Prognostic factors for survival in patients with thymic epithelial tumors. Thorac Cardiovasc Surg 2011;59:153-7. [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg 2010;89:245-52; discussion 252. [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of prognostic factors. Jpn J Thorac Cardiovasc Surg 2001;49:35-41. [PubMed]
- Attaran S, McCormack D, Pilling J, et al. Which stages of thymoma benefit from adjuvant chemotherapy post-thymectomy? Interact Cardiovasc Thorac Surg 2012;15:273-5. [PubMed]
- Evans TL, Lynch TJ. Role of chemotherapy in the management of advanced thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:41-50. [PubMed]
- Ansari M, Dehsara F, Mohammadianpanah M, et al. Treatment results and prognostic indicators in thymic epithelial tumors: a clinicopathological analysis of 45 patients. Iran J Med Sci 2014;39:341-9. [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [PubMed]
- Kumar N, Kumar R, Bera A, et al. Thymoma: clinical experience from a tertiary care institute from North India. J Cancer Res Ther 2013;9:235-9. [PubMed]
- Kim BK, Cho BC, Choi HJ, et al. A single institutional experience of surgically resected thymic epithelial tumors over 10 years: clinical outcomes and clinicopathologic features. Oncol Rep 2008;19:1525-31. [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [PubMed]
- Zhu G, He S, Fu X, et al. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys 2004;60:1113-9. [PubMed]
- Froudarakis ME, Tiffet O, Fournel P, et al. Invasive thymoma: a clinical study of 23 cases. Respiration 2001;68:376-81. [PubMed]
- Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91; discussion 1591-2. [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. [PubMed]
- Ruffini E, Guerrera F, Filosso PL, et al. Reply to Hamaji. Eur J Cardiothorac Surg 2015;48:340-1. [PubMed]
- Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. [PubMed]
- Rena O, Papalia E, Oliaro A, et al. Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma? Eur J Cardiothorac Surg 2007;31:109-13. [PubMed]
- Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59-66. [PubMed]
- Sakamoto M, Murakawa T, Konoeda C, et al. Survival after extended thymectomy for thymoma. Eur J Cardiothorac Surg 2012;41:623-7. [PubMed]





