Malignant pleural mesothelioma: an epidemiological perspective
This paper reviews the aetiology, distribution and projected future incidence of malignant mesothelioma. Asbestos exposure is the most thoroughly established risk factor. Debate continues regarding the relative importance of the different asbestos fibre types and the contribution of Simian virus 40 (SV40). Disease incidence varies markedly within and between countries. The highest annual rates of disease, approximately 30 case per million, are reported in Australia and Great Britain. The risk of disease increases with age and is higher in men. Time from asbestos exposure to disease diagnosis is on average greater than 40 years. Nonoccupational asbestos exposures contribute an increasing proportion of disease. With the exception of the United States, incidence continues to increase. In developed countries peak incidence is expected to occur before 2030.
Key words: Asbestos; epidemiology; incidence; malignant mesothelioma
Malignant mesothelioma is a tumour arising from the mesothelial lining of the pleura, peritoneum, pericardium and tunica vaginalis. Pleural mesothelioma is the most common of these, accounting for approximately 90% of disease (1,2). Patients commonly present with dyspnoea, chest wall pain and pleural effusion (3). Diagnosis is often made at an advance stage of disease and in untreated patients median survival is less than one year (4).
Disease aetiology
The association of mesothelioma with asbestos exposure is well established, with an aetiological fraction above 80% (5). Indeed, incidence of the disease prior to the widespread commercial production of asbestos was rare (6,7). The link was first demonstrated by Wagner et al. (8) in 1960, who described crocidolite asbestos exposure in 33 cases of mesothelioma in South Africa’s North Western Cape. Confirmation of the association came with eight case control studies conducted between 1965-75, which reported relative risk of exposure between 2.3 and 7.0 (9). Finally, McDonald (10) summarised 43 cohort mortality studies finding proportional mortality ratios for exposed subjects ranging from 2.5 to 102.3.
There are six types of asbestos that may be divided into two forms, serpentine and amphibole. The only serpentine type, chrysotile, also known as white asbestos, is made up of curled fibres and accounts for approximately 95% of all asbestos used worldwide (11). The amphibole group includes amosite, crocidolite, tremolite and anthophyllite (12). Their straighter, needle-like, friable fibres distinguish them from chrysotile. Of the amphiboles, amosite (brown asbestos) and crocidolite (blue asbestos) had the most industrial usage. The relative oncogenicity of the main asbestos fibre types, chrysotile in particular, is controversial. Hodgson and Darnton (13) examined average cumulative exposure in seventeen published cohorts and calculated a risk ratio of 1:100:500 for chrysotile, amosite and crocidolite respectively. Others argue that chrysotile is not carcinogenic and that the observed cases are due to contamination by the amphibole tremolite (14). Reviews of the epidemiological literature have yielded conflicting conclusions regarding the malignant potential of chrysotile (15-17). Studies of retained lung fibres in affected patients have reported increased odds ratios for amphibole fibres, as well as for chrysotile fibres alone (9). The evidence has been deemed sufficient by the World Health Organisation (WHO) to conclude that all types of asbestos cause cancer in humans (18).
The latency of mesothelioma, that is the time elapsed between first exposure to asbestos and the diagnosis of disease, is long. Investigators in New South Wales, Australia reported an average latency of 42.8 years for cases diagnosed between 1972 and 2004, without gender difference. Peritoneal disease had a significantly shorter latency than pleural disease. Longer latency periods were evident in more recent diagnoses (19). A second study, from Italy, reported a mean latency of 44.6 years in 2,544 cases diagnosed in the period 1993 to 2001, with shorter latency in those cases with occupational exposure (20). There is some evidence that disease latency has an inverse relationship with duration or degree of asbestos exposure. In a series of British Naval dockyard workers, Hilliard et al. (21) categorised the workers as continuously or intermittently exposed, finding a shorter latency in the more heavily exposed group (42 years, 95% CI, 39.0-45.0, versus 49.5 years, 95% CI, 48.2-50.9). Early studies reporting 20-30 year latency periods often involved insulation workers, a population with heavy asbestos exposure (22).
Although short or low-level asbestos exposures have been linked to the development of mesothelioma, the risk of disease demonstrates dose dependence. In the most closely studied asbestos-exposed population, residents of the Western Australian asbestos mining town Wittenoom, both mine workers and non-mining residents with greater intensity and duration of exposure had higher rates of disease (23,24). Length of employment has similarly been shown to increase mesothelioma risk in Norwegian insulation and asbestos-cement workers (25,26) Observation of high mesothelioma rates in the Cappadocian villages of Turkey has identified other potential aetiological factors (27). The regionally occurring fibrous mineral erionite has been detected in the villagers’ lungs, and is demonstrably carcinogenic in animal models (28). Pedigree analysis in affected families in this region also suggests a genetic susceptibility that is inherited in an autosomal dominant pattern, raising the possibility of a specific gene-environment interaction (29).
The DNA virus, Simian virus 40 (SV40), has been associated with malignant mesothelioma and has been suggested as a causal co-factor. The most likely route of human infection by SV40 is via contaminated polio vaccines until the late 1970s (30). SV40 inactivates tumour suppressor genes and has demonstrated oncogenic potential in animal experimentation (31). It has a predilection for mesothelial cells and is found in human mesothelioma specimens. It is not however present in all mesotheliomas (32), and PCRbased evidence for tumour infection may have been based on assays prone to false positive results (33). As such the role of SV40 in overall human mesothelioma incidence remains unclear.
Descriptive epidemiology
Worldwide malignant mesothelioma incidence has been rising since the mid 20th century. Analysis of mesothelioma mortality recorded in the WHO mortality database between 1994 and 2008 yielded an age-adjusted mortality rate of 4.9 per million, a mean age at death of 70 years and male to female ratio of 3.6:1 (34). There is marked heterogeneity in malignant mesothelioma incidence within and between countries. Table 1 outlines up-to-date incidence data in industrialised countries. Some of the most robust data comes from national registries in Australia and the United Kingdom, where age standardised incidence for 2009 was 29 per million of population in both countries. In Australia, male diagnoses dominate and more than 75% of newly diagnosed patients are aged 65 years or older. Incidence has been increasing each year since 16 cases were reported in 1980 (35). Comparable disease distribution is evident in the United Kingdom. Incidence in men has increased five-fold in the 30 years since 1980, and the age-specific incidence peaks at 75-79 years for women and 80-84 for men. In the United States, analyses of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program database estimate 2,500-3,000 cases per year, predominantly in elderly men. Furthermore the SEER incidence data suggest a plateau and subsequent decline in new mesothelioma cases since the years 2000-2005 (2,45). Crude incidence rates in a large proportion of Europe are in the range of 10-20 cases per million (22).
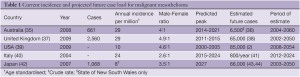
Full table
Global incidence of mesothelioma is likely to be significantly higher than mortality registries suggest due unreported cases occurring in developing counties. Park et al. (46) used the relationship between cumulative asbestos use and disease incidence in countries where both variables are published to estimate unreported cases in countries where only asbestos consumption is known. They describe a “hidden burden of disease” of approximately 39,000 cases in the 15-year period to 2008, predominantly in Russia, Kazakhstan, China, India and Thailand. Furthermore, mortality data in developed countries may underestimate true mesothelioma incidence due to inaccurate death certification (47) and undifferentiated International Classification of Diseases (ICD) codes for pleural malignancy until 1994 (48).
Exposure mapping within countries reveals high regional variability in incidence and mortality. In Italy, significant municipal clusters of disease have been identified close to asbestos cement industries, shipyards, oil refineries and petrochemical industries (49). Similarly in the UK, the highest mesothelioma mortality rates are recorded in areas with a history of ship building, such as Barrow-in-Frness, Plymouth, Portsmouth, Tyneside and Southampton (50). Regional variability is also evident in the registries contributing to the SEER Database. In 1998 the incidence ranged from 4.5 per million in Hawaii to 23.3 per million in Seattle- Puget Sound, an area historically associated with maritime industry (51). Small clusters of very high incidence have also been described secondary to environmental exposures. Villages in Turkey (52) and New Caledonia (53) with incidence rates above 1,000 per million are examples.
Given the role of asbestos in the aetiology of malignant mesothelioma, it is unsurprising that the relative risk of various occupational exposures have been extensively addressed in the epidemiological literature. Three waves of disease have been described. The first affected miners and millers of raw asbestos and in the manufacture of asbestos products. Former Wittenoom workers have been closely followed. Berry et al. (54) recently published 50-year follow-up in a cohort of 6,908 Wittenoom employees, reporting mesothelioma death rates of 4.7% and 3.1% for male and female workers respectively. Malignant mesothelioma accounted for 10% of known deaths in men and 8% in women in this cohort. A second wave of disease subsequently became evident in workers who used asbestos products in industry. Carpenters, plumbers, defence personnel, shipbuilders, and insulation installers are typical of the occupations affected (55).
Since the 1990s changing risk groups have been identified (56), prompting classification of a third wave of disease, in people with often unknown, short term or low level exposure to asbestos. Cited examples of these frequently non-occupational exposures include, domestic (family of asbestos workers), air pollution from nearby asbestos industry, or exposure to asbestos in place (buildings containing asbestos) (57). In Western Australia, increasing disease incidence attributed to exposure during home maintenance and renovation exemplifies the epidemiological shift (58). Non-occupational exposures of this type were found to account for 8.3% of cases in the period 1993- 2001 in Italy (59), but have been implicated in up to 30% of current presentations in the US and are predicted to account for an increasing proportion of disease (55).
Projections of future disease burden
An estimation of future mesothelioma disease burden was first undertaken using a birth-cohort model in British men (60). The model indirectly accounted for asbestos exposure and predicted a proportional hazard of mesothelioma mortality by age and year of birth. The projection predicted a peak of 2,700-3,300 deaths in Britain in the year 2020. This methodology was widely reproduced in different populations, but has subsequently been shown to overestimate peak incidence (61). Using improved modelling techniques, Price and Ware (2) further described reductions in incidence projections over time in the SEER data. Recent models allow more sophisticated estimation of asbestos exposure and mortality variation within birthcohorts (36,43,48).
With the exception of the United States, current predictions suggest peak mesothelioma incidence has not yet been reached (Table 1), and that in developed countries, this will occur in the second and third decades of the century. The late peak in Japan can be explained by a historical delay in heavy asbestos usage in that country (44). Future expected caseloads for each country or region are estimated from the annual incidence rate and expected peak year. They demonstrate that in industrialised nations alone, the disease is likely to affect hundreds of thousands of people in the next 50 years. High asbestos consumption in developing countries, particularly in Asia, is likely to cause additional future disease, however this is difficult to quantify (62).
Conclusions
Despite a clear understanding of malignant mesothelioma aetiology, the worldwide incidence continues to climb. The long latency of this disease and the continued distribution and consumption of asbestos products ensure that the toll of asbestos exposure will continue well into 21st century. The large future caseload underlines the ongoing importance of research directed towards early diagnosis and disease management.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Leigh J, Davidson P, Hendrie L, et al. Malignant mesothelioma in Australia, 1945-2000. Am J Ind Med 2002;41:188-201.
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88.
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62:ii1-ii19.
- Milano MT, Zhang H. Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol 2010;5:1841-8.
- McDonald JC, McDonald AD. The epidemiology of mesothelioma in historical context. Eur Respir J 1996;9:1932-42.
- Strauchen JA. Rarity of malignant mesothelioma prior to the widespread commercial introduction of asbestos: the Mount Sinai autopsy experience 1883-1910. Am J Ind Med 2011;54:467-9.
- Mark EJ, Yokoi T. Absence of evidence for a significant background incidence of diffuse malignant mesothelioma apart from asbestos exposure. Ann N Y Acad Sci 1991;643:196-204.
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71.
- McDonald JC. Epidemiology of malignant mesothelioma- -an outline. Ann Occup Hyg 2010;54:851-7.
- McDonald C. eds. Epidemiology of Work Related Diseases. London: BMJ Books, 2000.
- Virta RL. Worldwide asbestos supply and consumption trends from 1900 to 2000 (Open-file report): U.S. Dept. of the Interior, U.S. Geological Survey;2003:59.
- International Agency for Research on Cancer. Review of Human Carcinogens: C. Metals, Arsenic, Dusts and Fibres (IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans): World Health Organization.2012:213.
- Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg 2000;44:565-601.
- Britton M. The epidemiology of mesothelioma. Semin Oncol 2002;29:18-25.
- Kanarek MS. Mesothelioma from chrysotile asbestos: update. Ann Epidemiol 2011;21:688-97.
- Powers A, Carbone M. The role of environmental carcinogens, viruses and genetic predisposition in the pathogenesis of mesothelioma. Cancer Biol Ther 2002;1:348-53.
- Yarborough CM. Chrysotile as a cause of mesothelioma: an assessment based on epidemiology. Crit Rev Toxicol 2006;36:165-87.
- World Health Organisation (WHO). Elimination of asbestos-related diseases. Geneva: WHO, 2006.
- Hyland RA, Ware S, Johnson AR, et al. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health 2007;33:286-92.
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722-8.
- Hilliard AK, Lovett JK, McGavin CR. The rise and fall in incidence of malignant mesothelioma from a British Naval Dockyard, 1979-1999. Occup Med (Lond) 2003;53:209-12.
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379-87.
- Hansen J, de Klerk NH, Eccles JL, et al. Malignant mesothelioma after environmental exposure to blue asbestos. Int J Cancer 1993;54:578-81.
- Armstrong BK, de Klerk NH, Musk AW, et al. Mortality in miners and millers of crocidolite in Western Australia. Br J Ind Med 1988;45:5-13.
- Ulvestad B, Kjaerheim K, Martinsen JI, et al. Cancer incidence among members of the Norwegian trade union of insulation workers. J Occup Environ Med 2004;46:84-9.
- Ulvestad B, Kjaerheim K, Martinsen JI, et al. Cancer incidence among workers in the asbestos-cement producing industry in Norway. Scand J Work Environ Health 2002;28:411-7.
- Artvinli M, Bariş YI. Malignant mesotheliomas in a small village in the Anatolian region of Turkey: an epidemiologic study. J Natl Cancer Inst 1979;63:17-22.
- Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008;9:147-57.
- Roushdy-Hammady I, Siegel J, Emri S, et al. Geneticsusceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet 2001;357:444-5.
- Cutrone R, Lednicky J, Dunn G, et al. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res 2005;65:10273-9.
- Cicala C, Pompetti F, Carbone M. SV40 induces mesotheliomas in hamsters. Am J Pathol 1993;142:1524-33.
- Hirvonen A, Mattson K, Karjalainen A, et al. Simian virus 40 (SV40)-like DNA sequences not detectable in finnish mesothelioma patients not exposed to SV40-contaminated polio vaccines. Mol Carcinog 1999;26:93-9.
- López-Ríos F, Illei PB, Rusch V, et al. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet 2004;364:1157-66.
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-724C.
- Safe Work Australia. Mesothelioma in Australia: Incidence 1982 to 2008, Mortality 1997 to 2007. Canberra: Safe Work Australia. 2012.
- Clements M, Berry G, Shi J, et al. Projected mesothelioma incidence in men in New South Wales. Occup Environ Med 2007;64:747-52.
- Cancer Research UK. Mesothelioma statistics. 2012 [cited 2012]; Available online: http://www.cancerresearchuk.org/ cancer-info/cancerstats/types/Mesothelioma/.
- Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93.
- Surveillance Research Program of the National Cancer Institute. Cancer statistics review 1975-2009. Washington: National Cancer Institute. 2009.
- Marinaccio A, Binazzi A, Marzio DD, et al. Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer 2012;130:2146-54.
- Marinaccio A, Montanaro F, Mastrantonio M, et al. Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer 2005;115:142-7.
- Aoe K, Hiraki A, Fujimoto N, et al. The first nationwide survival analysis of Japanese mesothelioma patients from Vital Statistics of Japan. J Clin Oncol 20120;28:abstr e12007.
- Myojin T, Azuma K, Okumura J, et al. Future trends of mesothelioma mortality in Japan based on a risk function. Ind Health 2012;50:197-204.
- Murayama T, Takahashi K, Natori Y, et al. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am J Ind Med 2006;49:1-7.
- Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol 200;159:107-12.
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8.
- Okello C, Treasure T, Nicholson AG, et al. Certified causes of death in patients with mesothelioma in South East England. BMC Cancer 2009;9:28.
- Camidge DR, Stockton DL, Bain M. Factors affecting the mesothelioma detection rate within national and international epidemiological studies: insights from Scottish linked cancer registry-mortality data. Br J Cancer 2006;95:649-52.
- Fazzo L, De Santis M, Minelli G, et al. Pleural mesothelioma mortality and asbestos exposure mapping in Italy. Am J Ind Med 2012;55:11-24.
- McElvenny DM, Darnton AJ, Price MJ, et al. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79-87.
- Pinheiro GA, Antao VC, Bang KM, et al. Malignant mesothelioma surveillance: a comparison of ICD 10 mortality data with SEER incidence data in nine areas of the United States. Int J Occup Environ Health 2004;10:251-5.
- Metintas S, Metintas M, Ucgun I, et al. Malignant mesothelioma due to environmental exposure to asbestos: follow-up of a Turkish cohort living in a rural area. Chest 2002;122:2224-9.
- Baumann F, Maurizot P, Mangeas M, et al. Pleural mesothelioma in New Caledonia: associations with environmental risk factors. Environ Health Perspect 2011;119:695-700.
- Berry G, Reid A, Aboagye-Sarfo P, et al. Malignant mesotheliomas in former miners and millers of crocidolite at Wittenoom (Western Australia) after more than 50 years follow-up. Br J Cancer 2012;106:1016-20.
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603.
- Huncharek M. Changing risk groups for malignant mesothelioma. Cancer 1992;69:2704-11.
- Hillerdal G. Mesothelioma: cases associated with nonoccupational and low dose exposures. Occup Environ Med 1999;56:505-13.
- Olsen NJ, Franklin PJ, Reid A, et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Aust 2011;195:271-4.
- Mirabelli D, Cavone D, Merler E, et al. Non-occupational exposure to asbestos and malignant mesothelioma in the Italian National Registry of Mesotheliomas. Occup Environ Med 2010;67:792-4.
- Peto J, Hodgson JT, Matthews FE, et al. Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535-9.
- Segura O, Burdorf A, Looman C. Update of predictions of mortality from pleural mesothelioma in the Netherlands. Occup Environ Med 2003;60:50-5.
- Park EK, Takahashi K, Jiang Y, et al. Elimination of asbestos use and asbestos-related diseases: An unfinished story. Cancer Sci 2012;103:1751-5.





