Extrapleural pneumonectomy and extended pleurectomy/decortication for malignant pleural mesothelioma: the Memorial Sloan-Kettering Cancer Center approach
Introduction
Surgical management for malignant pleural mesothelioma (MPM) remains controversial, but because of the limitations of radiation and chemotherapy in this disease, surgery is still an important part of treatment for these patients (1-13). Operations for MPM can be categorized as those performed with either palliative or curative intent. Palliative procedures such as video-assisted thoracoscopy surgery (VATS) and talc pleurodesis control pleural effusions in patients whose overall medical condition precludes definitive resection (14). In such cases, thoracotomy and partial pleurectomy is indicated only when the pleural effusion is loculated and cannot be evacuated by VATS.
Curative intent operations include extrapleural pneumonectomy (EPP) and extended pleurectomy/decortication (P/D). This article provides a brief review of preoperative evaluation and the surgical technique for EPP and P/D (15-17).
Preoperative evaluation
Preoperative evaluation aims to determine whether the patient has potentially resectable tumor and sufficient cardiopulmonary reserve to undergo the planned operation (18-21).
Computed tomography (CT) of the chest and upper abdomen is the primary imaging study to assess the extent of the primary tumor and identify metastatic disease in the peritoneum or the contralateral lung and pleura. Magnetic resonance imaging (MRI) can help determine whether the primary tumor invades the chest wall or diaphragm but our experience suggests that MRI does not add significantly to CT in preoperative staging, especially in an era of routine coronal and sagittal CT imaging (22-27).
Positron emission tomography (PET) adds to CT in staging MPM. It identifies metastatic disease not detected by CT in approximately 10 percent of patients. The standardized uptake value (SUV) on PET is also an independent prognostic factor for overall survival and is useful in selecting patients for treatment (28-31). We use PET-CT routinely in the initial evaluation of patients with MPM.
Mediastinoscopy is frequently used by some institutions as a staging procedure before EPP because mediastinal nodal metastases (N2 disease) are an adverse prognostic factor in MPM, and because CT and PET are known to be inaccurate in detecting nodal disease (1,21,32-34). However, mediastinoscopy fails to identify N2 disease in some patients because the pattern of nodal metastases from MPM differs from that of lung cancer. Thus, patients may have metastases in the internal mammary or peri-diaphragmatic nodes without having involvement of the superior mediastinal nodes. In addition, N2 disease is only one of several important prognostic factors in MPM and does not uniformly identify all patients who have a poor prognosis. At the current time, we do not routinely perform mediastinoscopy as part of the initial staging evaluation for MPM. Mediastinoscopy is perhaps most helpful in identifying N2 disease in patients thought to have T1 tumors on CT who might not otherwise be considered for induction chemotherapy before surgical resection.
Laparoscopy has also been advocated as a staging maneuver before resection because it identifies trans-diaphragmatic tumor extension or intra-abdominal metastases (35,36). However, routine laparoscopy is not required in patients whose imaging studies show earlier stage tumors and no intra-abdominal abnormalities.
The assessment of cardiopulmonary reserve is a pivotal part of the preoperative evaluation for EPP. Pulmonary function testing (PFTs) should be performed, including a diffusion capacity (DLCO), because MPM patients who have had asbestos exposure often have underlying interstitial lung disease. When EPP is being considered, a quantitative ventilation/perfusion (V/Q) lung scan should also be done to help calculate the patient’s postoperative pulmonary function.
Most MPM patients are older and have medical co-morbidities, especially underlying cardiovascular disease (20,36-38). An EPP places patients at high risk for myocardial ischemia because of intra-operative blood loss and postoperative fluid shifts. Therefore, some form of stress testing should be considered preoperatively.
In summary, our routine preoperative evaluation of patients being considered for EPP includes a CT scan of the chest and upper abdomen, a PET-CT scan, complete PFTs, a quantitative V/Q scan, and usually, some form of stress testing. Additional evaluation including MRI, laparoscopy, mediastinoscopy or endobronchial ultrasound (EBUS) is performed selectively.
Surgical technique for EPP
A video in another section of this issue illustrates the technique for left EPP. This chapter describes the step-by-step technique for right and left EPP and for extended P/D.
Preparation, positioning and incision
Preoperatively, an epidural catheter is placed for postoperative analgesia. After induction of general anesthesia, a double lumen endotracheal tube is inserted. In addition to standard intra-operative monitoring (arterial line, pulse oximetry), a central venous catheter (CVC) is inserted to help manage peri-operative fluid shifts in patients undergoing EPP. A CVC is not generally used for extended P/D.
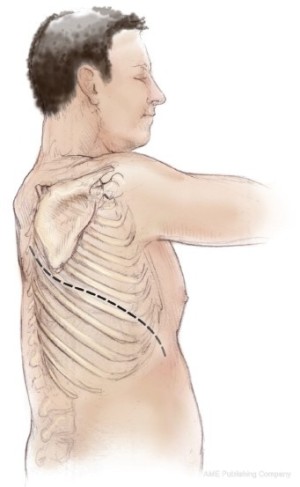
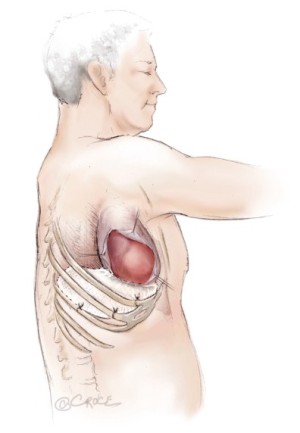
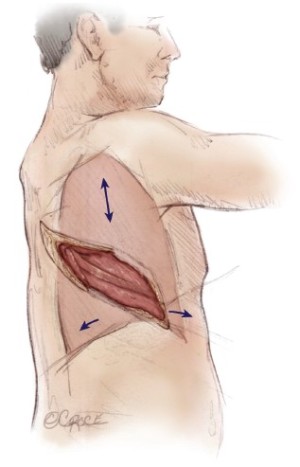
The patient is placed in the lateral decubitus position. Initial exploration is performed via a standard posterolateral thoracotomy incision. Once it is determined that the patient has resectable tumor without diffuse invasion of the chest wall, the incision is extended as an S-shaped thoracotomy with a long anterior component down towards the costal margin providing exposure for diaphragmatic resection and reconstruction (Figure 1). Some surgeons add a second small posterior thoracotomy incision at the level of the eleventh rib to provide exposure to the costophrenic sulcus (39) but this causes more pain and chest wall edema and does not significantly improve exposure. If postoperative hemithoracic radiation is planned there is no need to excise previous chest wall incisions, because the radiation treats potential tumor implants. Both the latissimus dorsi and the serratus anterior muscles are divided. Some authors recommend using a median sternotomy rather than a thoracotomy, especially for right sided resection (40). However, this approach does not provide as much exposure for resection and reconstruction of the posterior aspect of the diaphragm and is not used at Memorial Sloan-Kettering Cancer Center (MSKCC).
Technique of resection
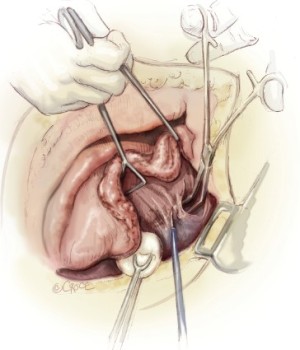
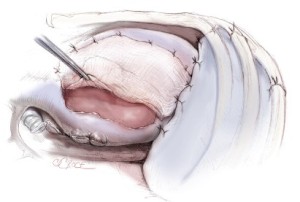
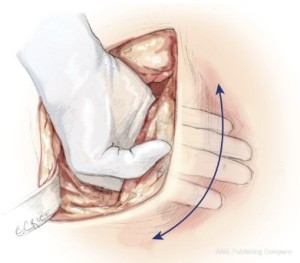
The sixth rib is excised to expose to the extrapleural plane. The intercostal muscles are carefully preserved for reclosure at the end of the operation. This approach is slightly lower than for a standard pulmonary resection to facilitate exposure of the diaphragm. Blunt dissection is performed in the extrapleural plane freeing the parietal pleura from the endothoracic fascia using a sweeping motion of the hand (Figure 2) up to apex of the chest, then down to the diaphragm, anteriorly to the pericardium, and posteriorly to the spine (Figure 3). Hemostasis is obtained as the dissection is performed to prevent substantial blood loss from the chest wall. The most effective hemostatic tool for this is the Tissue Link (Tissue Link Medical, Inc., Dover, NH). Once the parietal pleura is mobilized away from the chest wall, a chest retractor is inserted, and dissection continues under direct vision mobilizing the pleura circumferentially away from the mediastinum. On the left side, care is taken to identify the plane between the tumor and the adventitia of the aorta and the esophagus. On the right side, dissection along the superior vena cava must be performed very gently. In some patients, there is a clean plane of dissection between the mediastinal pleura and the pericardium. In others, this plane is obliterated and the anterior mediastinal pleura has to be resected en-bloc with the pericardium later in the operation. After the pleura and lung are completely mobilized in the upper half of the chest, exposing the superior and posterior aspects of the hilum, an en-bloc dissection of the subcarinal lymph nodes is performed for staging purposes and to expose the main-stem bronchus (Figure 4).
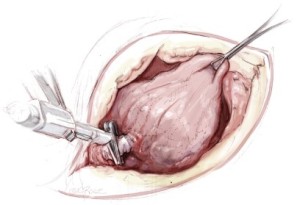
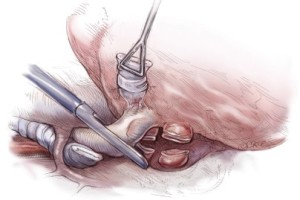
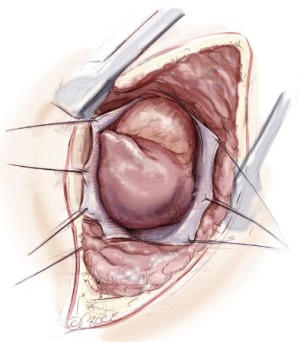
The diaphragmatic tumor is then resected. There is always a palpable “edge” laterally between the tumor and normal diaphragmatic muscle or peritoneum at the level of the costophrenic sulcus. This plane is developed and the tumor mobilized along its diaphragmatic surface by blunt dissection similar to a Kocher maneuver. Once mobilized from the posterior and lateral costophrenic angles, the tumor is rotated up into the thoracotomy incision, rolling it back upon itself, and placing strong traction on the diaphragm (Figure 5). If the involvement of the diaphragm is extensive, it is removed entirely, peeling it away from the peritoneum. If the involvement of the diaphragm is superficial, dissection can be carried through the diaphragmatic muscle using the electrocautery (Figure 5). Every effort is made not to enter the peritoneum because of the propensity of MPM to produce tumor implants. However, entry into the abdomen at the level at the central tendon is unavoidable because of the fusion of tissue planes in that area. The peritoneum is immediately reclosed as the central tendon is removed. The diaphragm is mobilized back to the pericardium medially.
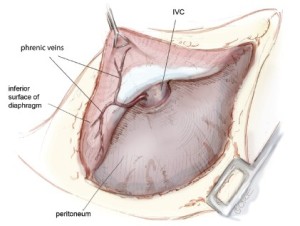
For right-sided resections, it is very important to identify the phrenic veins draining from the diaphragm directly into the inferior vena cava (IVC) (Figure 6). There are a variable number of such veins, which must be carefully mobilized, ligated and divided to avoid bleeding the IVC. These veins are not present on the left side of the diaphragm. If resection of the pericardium is required, it is entered only when the tumor has been mobilized as fully as possible from all other directions because traction on the pericardium causes arrhythmias and hemodynamic instability (Figure 7).
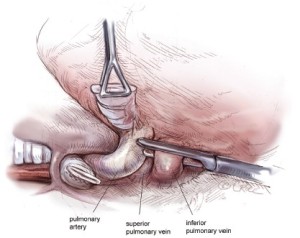
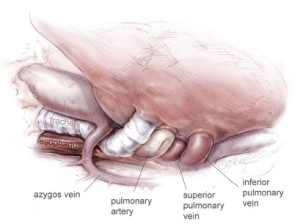
The hilar structures are divided in whatever sequence is technically easiest and requires the least manipulation of the large tumor mass, usually the main-stem bronchus first (Figure 8), then the inferior pulmonary vein (Figure 9), the superior pulmonary vein, and lastly the main pulmonary artery (Figure 10) If the pericardium is resected, it is gradually opened as this portion of the dissection is carried out. Traction sutures are placed on the pericardium to prevent it from retracting toward the opposite hemithorax thereby minimizing changes in the position of the heart and avoiding hemodynamic instability. The specimen consisting of pleura, lung, and diaphragm, with or without pericardium, is removed en-bloc. Dissection of the paratracheal lymph nodes if the operation is on the right, or of the aorto-pulmonary window nodes if the operation is on the left, is performed for staging purposes.
Reconstruction of the diaphragm and pericardium
Reconstruction of the diaphragm is performed using a 2 mm thickness Gore Tex patch (W.L. Gore & Associates, Flagstaff, AZ). Laterally, the prosthetic patch is secured with #2 Vicryl sutures around the ribs. Posteriorly, it is sutured to the crus or gently tacked with fine sutures to the wall of the esophagus. Medially it is sewn to the edge of the pericardium with 0-Prolene interrupted sutures (Figure 11). It is extremely important to place the diaphragmatic reconstruction at the same level as the native diaphragm, namely at the tenth intercostal space posteriorly and at the eighth and ninth intercostal spaces anteriorly and laterally. Placing the reconstruction any higher than this makes it difficult to deliver adjuvant radiation safely, especially to the posterior costophrenic sulcus, and increases the risk of radiation hepatitis after right-sided and radiation gastritis after left-sided resections. Although some surgeons recommend reconstructing the diaphragm with a latissimus dorsi reverse flap (41), this technique is more complex and does not provide the stable reconstruction needed to facilitate postoperative radiation.
If the pericardium is resected, it is reconstructed with absorbable mesh to prevent cardiac herniation into the empty hemithorax, and facilitate postoperative radiation to the hemithorax by maintaining the heart in a central position (Figure 12). Some surgeons prefer a 1 mm thickness Gore Tex fenestrated patch for pericardial reconstruction. However, this is more difficult to size than absorbable mesh for the pericardial defect and is associated with a risk of epi- and pericarditis (42). A chest tube, usually a 32 French right angle tube, is inserted and placed on the diaphragmatic reconstruction to drain the blood that inevitably oozes from the chest wall dissection. The thoracotomy incision is closed, carefully reapproximating the intercostal muscles to prevent leakage of fluid from the pleural space.
Important aspects of postoperative care
Fluid management is critical after EPP. Ongoing fluid shifts during surgery make hemoglobin an unreliable guide to transfusion. Transfusing the patient according to measured intra-operative blood loss is more appropriate and, in our experience, will avoid peri-operative hypotension. Gradual intravascular equilibration during the first few days postoperatively usually requires additional transfusions. Monitoring of the central venous pressure during the first 24 hours postoperatively is helpful in assessing fluid management. As for any pneumonectomy, administration of intravenous crystalloid solution should be minimized.
The chest tube is placed to gravity drainage using a balanced drainage system to equilibrate the mediastinum. Leaving the chest tube in for 48 hours until the drainage becomes serosanguinous avoids the accumulation of a large hemothorax. A purse string suture should be placed around the chest tube and tied upon removal of the tube to prevent leakage of pleural fluid from the chest tube site.
Atrial fibrillation occurs in approximately one-third of patients after EPP. We routinely start diltiazem prophylactically on the first postoperative day and continue that for up to six weeks. Careful attention should be paid to the position of the mediastinum after the chest tube has been removed. Because the pleural space usually fills with fluid faster than air is resorbed from it, the mediastinum often shifts away from the operated side during the first five days postoperatively. Mediastinal shift can cause refractory atrial arrhythmias, which respond immediately to aspiration of air from the pleural space, but are not controlled by medication. Prophylactic aspiration of the pleural space performed as soon as the tracheal silhouette is seen to shift even slightly on chest X-ray will prevent these arrhythmias and also relieve the sense of dyspnea experienced by patients when the mediastinum is compressed. Aspiration of the pleural space is performed by inserting a catheter or spinal needle into the first or second intercostal space at the mid-clavicular line under sterile conditions with local anesthesia while the patient is sitting upright. The catheter is attached to a three-way stopcock and a 50 mL syringe. No more than 500 mL of air and/or fluid is aspirated at one time to avoid rapid shift of the mediastinum.
Respiratory insufficiency (atelectasis, retained secretions, pneumonia and acute lung injury) is the most common complication and great attention is given to early ambulation and to the maintenance of pulmonary toilet.
Surgical technique for extended P/D
All of the initial steps for extended P/D are identical to those performed for EPP, including the thoracotomy incision, resection of the 6th rib, extrapleural dissection along the chest wall and mediastinum and mobilization of the diaphragm. Systematic mediastinal lymph node dissection is also performed. Once the parietal pleura has been fully mobilized, it is incised entering the pleural space. The parietal pleura is mobilized away from the underlying lung, also removing all areas of visceral pleural tumor. In patients who have extensive visceral pleural disease including involvement of the fissures, this part of the dissection is extremely tedious and leaves extensively or completely denuded lung upon completion of the operation. Reconstruction of the diaphragm and pericardium is performed as outlined above for an EPP. It is especially important for the diaphragmatic prosthesis to sit at the same level as the native diaphragm and to be taut because any rise in the patch reconstruction not only compromises postoperative radiation but also produces symptomatic lower lobe atelectasis postoperatively.
Unlike EPP, closure of the thoracotomy incision after extended P/D does not require reapproximation of the intercostal muscles since there is no fluid accumulating in the pleural space. At least two chest tubes (or even three) are used to evacuate the pleural space and aid in re-expanding the lung.
Postoperatively, chest tube output can be considerable for the first 72 hours because of the extrapleural dissection. Patients may require additional intravenous fluids to compensate for this. The other peri-operative care issues that arise after EPP (e.g., rebalancing the mediastinum) do not occur after extended P/D. However, both EPP and extended P/D patients require meticulous respiratory care during the first 5 to 7 days postoperatively.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65.
- Martin-Ucar AE, Nakas A, Edwards JG, et al. Case-control study between extrapleural pneumonectomy and radical pleurectomy/decortication for pathological N2 malignant pleural mesothelioma. Eur J Cardiothorac Surg 2007;31:765-70; discussion 770-1.
- de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;133:111-6.
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92;discussion 1692-3.
- Aigner C, Hoda MA, Lang G, et al. Outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;34:204-7.
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6,626.
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64; discussion 264.
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24.
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703.
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6.
- Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011;40:298-303.
- Yan TD, Cao CQ, Boyer M, et al. Improving survival results after surgical management of malignant pleural mesothelioma: an Australian institution experience. Ann Thorac Cardiovasc Surg 2011;17:243-9.
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association for the Study of Lung Cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9.
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer committee and the International Mesothelioma Interest Group. J Thorac Oncol 2011;6:1304-12.
- Chang MY, Sugarbaker DJ. Extrapleural pneumonectomy for diffuse malignant pleural mesothelioma: techniques and complications. Thorac Surg Clin 2004;14:523-30.
- Rusch VW. Technique of extrapleural pneumonectomy for malignant pleural mesothelioma. In: Pearson FG, Patterson GA, Cooper J, et al. eds. Thoracic Surgery, Third ed. New York: Churchill Livingstone, 2008:1186-93.
- Rusch VW. Diffuse Malignant Mesothelioma. In: Shields TW, LoCicero J, Reed CE, et al. eds. General Thoracic Surgery, 7th ed. Philadelphia: Lippincott, Williams & Wilkins, 2009:847-59.
- Sugarbaker DJ, Heher EC, Lee TH, et al. Extrapleural pneumonectomy, chemotherapy, and radiotherapy in the treatment of diffuse malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1991;102:10-4; discussion 14-5.
- Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1996;111:815-25; discussion 825-6.
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804.
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5.
- Rusch VW, Godwin JD, Shuman WP. The role of computed tomography scanning in the initial assessment and the follow-up of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1988;96:171-7.
- Patz EF Jr, Shaffer K, Piwnica-Worms DR, et al. Malignant pleural mesothelioma: value of CT and MR imaging in predicting resectability. AJR Am J Roentgenol 1992;159:961-6.
- Patz EF Jr, Rusch VW, Heelan R. The proposed new international TNM staging system for malignant pleural mesothelioma: application to imaging. AJR Am J Roentgenol 1996;166:323-7.
- Heelan RT, Rusch VW, Begg CB, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999;172:1039-47.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95.
- Cao C, Krog Andvik SK, Yan TD, et al. Staging of patients after extrapleural pneumonectomy for malignant pleural mesothelioma--institutional review and current update. Interact Cardiovasc Thorac Surg 2011;12:754-7.
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2003;126:11-6.
- Erasmus JJ, Truong MT, Smythe WR, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg 2005;129:1364-70.
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography predicts survival in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2006;132:763-8.
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17.
- Pilling JE, Stewart DJ, Martin-Ucar AE, et al. The case for routine cervical mediastinoscopy prior to radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2004;25:497-501.
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7.
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2008;136:605-10.
- Conlon KC, Rusch VW, Gillern S. Laparoscopy: an important tool in the staging of malignant pleural mesothelioma. Ann Surg Oncol 1996;3:489-94.
- Rice DC, Erasmus JJ, Stevens CW, et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005;80:1988-92; discussion 1992-3.
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95.
- de Perrot M, McRae K, Anraku M, et al. Risk factors for major complications after extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Thorac Surg 2008;85:1206-10.
- Politi L, Scanagatta P, Salani A, et al. Double unilateral thoracotomy for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2004;45:591-2.
- Edwards JG, Martin-Ucar AE, Stewart DJ, et al. Right extrapleural pneumonectomy for malignant mesothelioma via median sternotomy or thoracotomy? Short- and long-term results. Eur J Cardiothorac Surg 2007;31:759-64.
- Bedini AV, Andreani SM, Muscolino G. Latissimus dorsi reverse flap to substitute the diaphragm after extrapleural pneumonectomy. Ann Thorac Surg 2000;69:986-8.
- Byrne JG, Karavas AN, Colson YL, et al. Cardiac decortication (epicardiectomy) for occult constrictive cardiac physiology after left extrapleural pneumonectomy. Chest 2002;122:2256-9.