Associated bare stenting of distal aorta with a Djumbodis® system versus conventional surgery in type A aortic dissection
Introduction
In the current era, it has become technologically possible to extend the repair of Stanford type A dissection (TAD) beyond the arch in the initial surgery by using hybrid prostheses or stents to cover the descending aorta during circulatory arrest (1-5). However, there is still a paucity of comparative studies to support this strategy compared to a more conventional hemiarch repair (6). Furthermore, exposing acute patients to an additional procedure, compared to standard treatment, is sometimes perceived as excessively high-risk in the context of an aortic disease with an already poor prognosis (7). Among current available devices, the Djumbodis® device system (DDS) is a novel technological advancement to manage TADs. It utilizes a bare metal stent that conforms to the shape of the aorta to compress the false lumen and still maintains the patency of the supra aortic vessels. Since CE approval in 2000 for the treatment of aortic dissections (type A or B), current data in the literature have demonstrated both favorable and less favorable results (8-10). However, comparative studies are still lacking.
Therefore, we conducted this post market clinical follow-up study to determine the effects of antegrade bare stenting of the dissected aorta beyond the distal anastomosis with a DDS, compared to conventional surgery in patients operated on for TAD. Early outcomes, overall mortality from aortic cause and late aortic events, including re-interventions, were examined.
Methods
Patients
We studied a consecutive cohort of 134 patients operated on under the direction of two senior surgeons (TC, AC) in two participating centers (respectively Amiens University Hospital, France and Meshalkinclinic, Novosibirsk, Russia) between 2005 and 2015. Patients were distributed into study and control groups according to the treatment received: conventional surgery with bare stenting of the distal aorta with a DDS downstream to the distal anastomosis (DJ group, n=42) or without (control group, n=92).
Surgical techniques
All patients were operated with cardiopulmonary bypass (CPB). CPB was connected according to one of the configurations: femoral vein—femoral artery, right atrium—subclavian artery or right atrium—the ascending aorta. “Conventional surgery” describes interventions on the ascending aorta and aortic arch, including aortic root replacement or valve sparing operations with aortic arch repair (hemiarch technique) or total arch replacement. The distal anastomosis was performed during deep or moderate hypothermic circulatory arrest with antegrade or retrograde cerebral perfusion. DDS was deployed in the true lumen of the aortic arch and the descending thoracic aorta by using all precautions required to avoid hazardous or harmful situations for patients. Such cautionary measures included respecting recommendations of use established by the manufacturer, and by promoting gentle balloon inflation with a certain volume of saline according to the calculation table and preoperative aortic diameter on computed tomography (CT)-scan images, as described elsewhere (11). The position and deployment of the stent was controlled by endoscope or transesophageal echocardiography. Finally, aortic arch and ascending aorta reconstructions were performed.
Endpoints
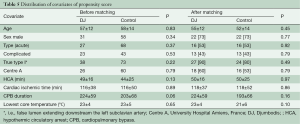
Study endpoints included operative (30-day) mortality from any cause, overall mortality from aortic causes, late aortic event-free survival and the need for further aortic or vascular re-interventions. For each group, operative mortality was monitored with CUSUM control charts and reported into an Excel Sheet (Microsoft Corp, Redmond, Washington, USA) designed to construct a cumulative failure graph, where boundary lines were calculated for p0 of 15% and p1 of 20%, and for a type I error equal 0.05 and a type II error equal 0.2. All other statistics were performed with SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means ± standard deviation. A Kolmogorov-Smirnov test was used to accept or reject normal distribution and comparisons between groups were performed with a two-tailed probability t-test or Wilcoxon test as appropriate. Category variables were analyzed using the chi-square test, and covariates influencing hospital mortality were identified using a logistic-stepwise regression analysis. Freedom from aortic events, death from aortic cause and re-interventions were assessed using the Kaplan-Meier method, and differences between groups were determined using the log-rank analysis. Because the allocation of patients to the treatment groups depended on preoperative case assessment and could have influenced outcomes (12), we also conducted a propensity-matched analysis of late results in early survivors. This compared patients who received an additional stenting of the distal aorta with a DDS, to those treated with conventional surgery alone. Pairing of patients was based on the estimated propensity score, calculated from a logit model which included age, sex, acuity of dissection (acute or subacute versus chronic), clinical presentation (complicated versus uncomplicated), extent of the false lumen, and participating center. Using the estimated logit function, we randomly selected a discharged patient from the control group to be matched with a discharged patient from the DJ group within a caliper of 0.035. Selection of control cases was performed without replacement matching.
Definitions
TAD was defined as any thoracic aortic dissection or intramural hematoma involving the proximal aorta and presenting for acute and chronic forms within 14 days of symptom onset and after 14 days, respectively.
Aortic event-free survival: in the setting of this study, aortic event-free survival referred to survivors without aortic complications (including sudden death, reoperation or diagnosis of a critical or evolving aneurysm).
Critical or evolving aneurysm: any increase of aortic diameter to above 5 cm or 2.5 cm2/m2 at the level of the thoracic or abdominal aorta; 4.5 cm in patients with Marfans disease; or any increase in diameter during follow-up of more than 0.5 cm within a year.
Re-intervention: Vascular re-interventions included any open surgery or endovascular procedures on the aortic branches or more distal arteries, as well as bypass performed to perfuse an ischemic limb or visceral territory. Other re-interventions included surgery to treat the consequences of malperfusion (bowel resection or amputation). Re-interventions for excessive bleeding or postoperative cardiac tamponade were excluded.
Results
Preoperative and intra-operative data
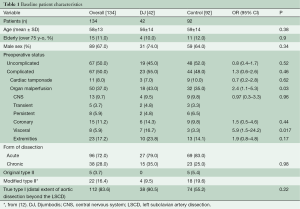
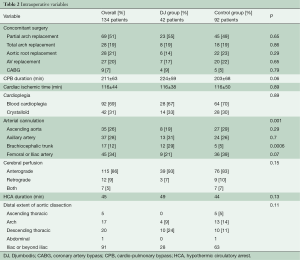
Table 1 summarizes preoperative data from patients before and after distribution into treatment groups. All patients with an original Type II aortic dissection and almost all patients with a modified Type II aortic dissection (12) were in the control group, which demonstrated fewer cases of lower body malperfusion (OR =2.4, P=0.03). In particular, preoperative visceral malperfusion was significantly more frequent in the DJ group (OR =5.9, P=0.02). However, preoperative cardiac tamponade was more frequent in the control group (OR =0.7, P=0.62). The overall picture was that more patients in the DJ group presented with complications at admission (OR =1.3; P=0.46). Concerning the extent of aortic repair (Table 2), a tendency towards more arch replacements (P=0.7) but fewer aortic root replacements (P=0.3) was observed in the DJ group, compared to the control group.
Full table
Full table
Early outcomes
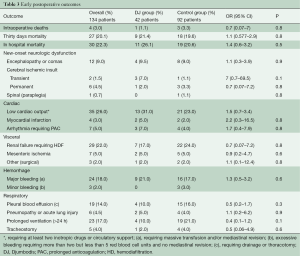
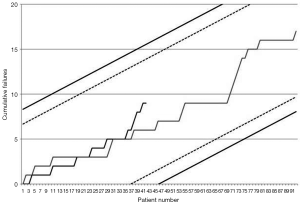
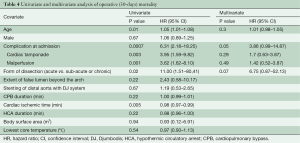
Early outcomes are summarized in Table 3. Operative mortality was 21.4% and 17.6% in the DJ group and control group respectively (P=0.9) and was within statistical control for both groups in this series with a 95% certitude (Figure 1). There was one case of device-related operative death due to the rupture of a severely kinked descending aorta in an elderly female patient, in whom the unfavorable aortic anatomy had not been recognized on preoperative CT-angiogram. Despite the fact that an immediate repair was possible under a second circulatory arrest, brain death was diagnosed on the third postoperative day. Univariate analysis (Table 4) showed that covariates of hospital mortality were age (P=0.02), complications at admission (P=0.0007) including cardiac tamponade (P=0.003) or any malperfusion (P=0.001), and acuity of dissection (acute vs. subacute or chronic) (P=0.02), but not the extent of the false lumen or the use of a Djumbodis system. In multivariate analysis (Table 4), the presence of any complication (cardiac tamponade or malperfusion) at admission was the only independent predictor of operative mortality (P=0.05).
Full table
Full table
Late outcomes
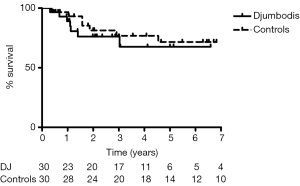
Sixty patients were included in the matched survivor cohorts study for a median follow-up of 7 years. Covariates of propensity matching score were equally balanced among groups (Table 5). The aortic event-free survival at 7 years for early survivors was 77%±10% and 48%±11% in the matched DJ group and control group, respectively (HR =0.66) (Figure 2). Freedom from aortic death was 91%±5% and 83%±8% in the matched DJ group and control group, respectively (HR=0.77). Therefore, at the end of follow-up, late mortality from aortic cause was 10% and 20% in the matched DJ group and control group respectively (RR =0.5, 95% CI, 0.14–1.82). Hazard ratio endpoints for the overall unmatched and matched cohorts (DJ group vs. control) are shown in Table 6.
Full table

Full table
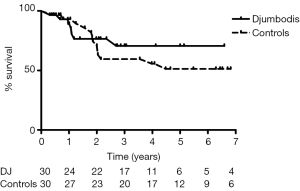
Actuarial freedom from aortic or vascular re-interventions was 71%±10% and 67%±9% in the matched DJ group and control group, respectively (Figure 3). For the matched DJ group, non-lethal re-interventions consisted of carotid reconstruction (n=1), kidney bypass from iliac artery (n=1) and open major vascular surgery on descending thoracic aorta (n=4), including one case of septic mycotic pseudo-aneurysm. Two patients died following redo surgery, one after aortic valve replacement due to severe aortic insufficiency, and one after extensive open thoraco-abdominal aneurysm repair nine years after prior surgery for acute TAD. For the matched control group, re-interventions consisted of renal artery stenting (n=1), repair of the dehiscence of the upper anastomosis after segment I plus hemi-arch replacement (n=1), valve replacement and arch replacement due to severe aortic valve regurgitation together with a dissecting aneurysm, and open major vascular surgery in five cases for a dissecting aneurism of the descending thoracic aorta (n=3) or of the thoraco-abdominal aorta (n=2). Although there were no deaths immediately following redo surgery, one patient eventually died within the same year from the consequences of subsequent paraplegia.
Discussion
Our study is a typical post-market clinical follow-up study which follows guidance document MEDDEV 2.12/2 rev 2 (Jan 2012) from the European Commission. It aims to enlighten ad-hoc committees for reimbursement of the device by health care assurance systems on the basis of the evaluation of device’s safety and efficiency. Our results demonstrate that there is no increase in early mortality due to the use of a DDS as compared to conventional surgery in TAD. Indeed, the early mortality observed in this series remained within the predefined acceptable limits of both participating centers. Because we have not performed any distal aortic stenting in the most favorable anatomical forms of TAD (i.e., De Bakey type II) and as updated recommendations on the use of the DDS included documented malperfusion of the lower body in order to improve blood flow in the distal true lumen (11), a low mortality in that group was not expected. This was due to a high proportion of patients who presented with life-threatening complications, especially end stage organ malperfusion (P=0.03). This well-known bias has already been encountered in previous studies that proposed an extensive surgical repair of acute DeBakey I aortic dissection (13). On the other hand, the risk of device-related death, which remained rare in this series, might have been balanced by potentially life-saving stenting of the descending aorta (14,15). This hypothesis can be hardly documented in this retrospective study, though we have observed that fewer patients required hemodialysis postoperatively, possibly due to better renal perfusion in the DJ group (OR =0.7; 95% CI, 0.07–7.2).
In any instance, the use of DDS system has not worsened early prognosis in the treated group, despite it being an uncovered balloon expandable stainless steel stent, which is perhaps the most feared device for use in type A acute dissections (6). Therefore, the concept of treating the descending aorta with endovascular devices at the same time of conventional surgery (1,2,4,16) is also supported by the present series, which is based on antegrade insertion of the stent during circulatory arrest. Interestingly, retrograde insertion of a Djumbodis® bare stent in the descending aorta following repair of acute DeBakey I aortic dissection has also been tested with outcomes superior to conventional surgical repair (16).
This series is the first to present a comparative sequential analysis of early outcomes between two alternative surgical strategies for acute TADs. CUSUM control charts of failure rate (17) are a powerful method for monitoring progress and its usage is increasing in a range of surgical fields. Amongst the reasons for this increasing interest is the fact that the CUSUM method is an intuitive form of sequential analysis, which provides sensitive, real-time monitoring with the potential for early detection of deteriorating trends in performance, even in case series with modest sample sizes. The key point of the method is the choice of adequate alert and alarm boundary lines in order to detect any process alteration that, when related to an excessive failure rate, might necessitate stopping patient recruitment to allow for further device investigations or for retraining of operators. By fixing a range of acceptable procedural risk between 15% and 20%, we ought to reflect that we admitted any patient with a type A acute aortic dissection for surgery within hours of diagnosis, therefore accepting to operate on even the most compromised cases. As a result, the observed operative mortality in this series stayed below the average mortality reported by the IRAD study group and by some other European large volume centers (18,19). Though this result was anticipated due to the proportion of cases with a subacute and chronic form of aortic dissection, which were included in the present series, it gives us reasonable confidence into the early safety of the device for extensive surgical repair of TAD.
We have used matched cohorts of survivors to compare late results between patients who have been treated with and without stenting of the aorta downstream to the distal anastomosis. Due to the large spectrum of clinical presentation of TAD that may have influenced survival, we have adopted one-to-one propensity score matching based on sex, age, acuity of dissection (acute vs. subacute or chronic) and clinical presentation (complicated vs. uncomplicated) to minimize potential bias. Because the decision to stent also depended on the surgeon on duty in this study, we considered the participating center as an obligatory covariate according to McMurry’s recommendations for building a propensity score (20). We did not consider introducing more variables into the scoring model to avoid excluding too many patients from the study. Taking into account these considerations, late mortality from aortic causes was reduced for matched early survivor patients who benefited from additional stenting of the distal aorta with a DJ device as compared to controls (RR =0.5; 95% CI, 0.14–1.82). We also observed that fewer aortic events occurred during follow-up in the DJ group (HR =0.66; 95% CI, 0.27–1.57). Although there was a similar rate of re-interventions in both groups from matched cohorts, three re-interventions in the DJ group did not involve the aorta itself and were only indicated to treat residual organ malperfusion. No device structural failure was observed during the study period. However, one patient in the DJ group had to undergo a total replacement of the descending aorta with a homograft one year postoperatively, because of a mycotic pseudo-aneurysm at the distal end of the stent. This illustrates that the device itself introduces a new hazard for re-intervention in the postoperative course.
Limitations
The current study focused on clinical results and did not examine remodeling of the dissected aorta after stenting. This issue has been already addressed elsewhere (11). Therefore, it is not possible to relate clinical issues to the faith of the false lumen in either group. As in any retrospective study, it is possible that some selection biases have not been taken into consideration, though propensity scoring and patients matching were performed by a blinded co-author (JN).
Conclusions
In the setting of this study, operative mortality was not influenced by antegrade stenting of distal aorta with a Djumbodis® device system as compared to conventional surgery alone for TAD. We observed a tendency towards better organ perfusion in the DJ group in the postoperative course, and more aortic events and subsequently more deaths from aortic cause in the control group during late follow-up. The rate of aortic or vascular re-interventions, however, was identical in matched cohorts of early survivors. With longer follow-up as well as larger patient cohorts, these results may support the hypothesis that more extensive stenting of the descending and abdominal aorta (optionally via a retrograde route in a second endovascular procedure) might improve organ perfusion and long-term outcomes. Our data can be valuable to any further post market clinical evaluation.
Acknowledgements
All authors wish to thank Annie Delbaere for her participation in secretary and database management.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jakob H, Dohle DS, Piotrowski J, et al. Six-year experience with a hybrid stent graft prosthesis for extensive thoracic aortic disease: an interim balance. Eur J Cardiothorac Surg 2012;42:1018-25. [Crossref] [PubMed]
- Haverich A. Aortic arch replacement with frozen elephant trunk-when not to use it. Ann Cardiothorac Surg 2013;2:592-6. [PubMed]
- Ouzounian M, LeMaire SA, Coselli JS. Open aortic arch repair: state-of-the-art and future perspectives. Semin Thorac Cardiovasc Surg 2013;25:107-15. [Crossref] [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4. [Crossref] [PubMed]
- Di Eusanio M, Di Bartolomeo R. The Sun procedure-a new paradigm of treatment in DeBakey type 1 acute aortic dissection? Ann Cardiothorac Surg 2013;2:629-30.
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455-74. [Crossref] [PubMed]
- Sultan I, Szeto WY. Decision making in acute DeBakey I aortic dissection: Balancing extensive arch reconstruction versus mortality. J Thorac Cardiovasc Surg 2016;151:349-50. [Crossref] [PubMed]
- Czerny M, Stöhr S, Aymard T, et al. Effect on false-lumen status of a combined vascular and endovascular approach for the treatment of acute type A aortic dissection. Eur J Cardiothorac Surg 2012;41:409-13. [Crossref] [PubMed]
- Ius F, Vendramin I, Mazzaro E, et al. Transluminal stenting in type A acute aortic dissection: does the Djumbodis system have any impact on false lumen evolution? Ann Thorac Surg 2010;90:1450-6. [Crossref] [PubMed]
- Wong RH, Yu SC, Lau RW, et al. Delayed stent deformity and fracture of Djumbodis dissection system. Ann Thorac Surg 2014;97:e17-20. [Crossref] [PubMed]
- Caus T, Houbert-Janssens A, Gaubert JY, et al. Early experience with the DJUMBODIS system: what did we observed, what can we expect? Part 2. Angiol Sosud Khir 2014;20:61-73. [PubMed]
- Tsagakis K, Tossios P, Kamler M, et al. The DeBakey classification exactly reflects late outcome and re-intervention probability in acute aortic dissection with a slightly modified type II definition. Eur J Cardiothorac Surg 2011;40:1078-84. [PubMed]
- Omura A, Miyahara S, Yamanaka K, et al. Early and late outcomes of repaired acute DeBakey type I aortic dissection after graft replacement. J Thorac Cardiovasc Surg 2016;151:341-8. [Crossref] [PubMed]
- Massmann A, Kunihara T, Fries P, et al. Uncovered stent implantation in complicated acute aortic dissection type B. J Thorac Cardiovasc Surg 2014;148:3003-11. [Crossref] [PubMed]
- Mastroroberto P, Chello M, Jannelli G, et al. Uncovered stent-graft in the treatment for residual patent false lumen after surgical repair for acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2011;12:202-4. [Crossref] [PubMed]
- Hofferberth SC, Newcomb AE, Yii MY, et al. Hybrid proximal surgery plus adjunctive retrograde endovascular repair in acute DeBakey type I dissection: superior outcomes to conventional surgical repair. J Thorac Cardiovasc Surg 2013;145:349-54; discussion 354-5. [Crossref] [PubMed]
- Noyez L. Control charts, Cusum techniques and funnel plots. A review of methods for monitoring performance in healthcare. Interact Cardiovasc Thorac Surg 2009;9:494-9. [Crossref] [PubMed]
- Gariboldi V, Grisoli D, Kerbaul F, et al. Long-term outcomes after repaired acute type A aortic dissections. Interact Cardiovasc Thorac Surg 2007;6:47-51. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- McMurry TL, Hu Y, Blackstone EH, et al. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg 2015;150:14-9. [Crossref] [PubMed]





