Hemodynamics of the aortic valve and root: implications for surgery
The aortic valve divides the left ventricular outflow tract (LVOT) from the ascending aorta and its normal or abnormal function has a great impact on regulating physiological hemodynamic parameters. It is well known that the presence of aortic valve stenosis or insufficiency results in impaired hemodynamics with direct and dire consequences on the left ventricle. On the other hand, when normal, the valve and the root have long been perceived as a type of passive gate dividing the left ventricle from the ascending aorta and preventing any reversal of flow, with minimal effect on valve hemodynamics. However, two points need to be stressed. First, the aortic valve has to be considered a complex unit along with the other components of the aortic root, the sinuses and the sino-tubular (ST) junction (1). Second, the particular shape of the aortic root, with its narrowing at the ST junction and the direct continuity of the sinus wall with the valve leaflets, makes perfect control of the closing mechanism of the aortic cusps possible. It is well known that the vortices forming inside the sinuses due to the narrowing of the ST junction are of paramount importance in regulating the closing mechanism of the valve leaflets (2). Although Leonardo da Vinci understood and illustrated these vortices long ago (3,4), only quite recently has the sophisticated relationship among the various components of the aortic root in controlling valve motion been demonstrated experimentally (5). During isovolumetric contraction of the left ventricle, pressure is transmitted through the inter-leaflet triangle up to the commissure in such a way that the valve starts to open even before forward flow has started. Next, as soon as the blood exits the ventricle, the regions of flow closer to the wall, being slower, curl down into the sinuses and already start closing the aortic valve. The main result of all these complex mechanisms is to keep the stress on the valve leaflet to a minimum (1), allowing a life-long valve durability.
Although almost everything with regard to the diastolic function of the aortic root is known, much less is clear about its systolic function or about the possibility that the aortic root, with its particular shape, might also regulate the opening of the aortic valve (6). This year our group was able to shed some light on the role of the aortic root in regulating the systolic function of the aortic valve (7). More specifically, we were able to show, for the first time, that the shape of the root, namely the presence of the sinuses, coupled with the pulsatility of the flow, was important in guaranteeing complete opening of the valve and thereby absence of a trans-valvular gradient. In this respect, root compliance seemed to be less important.
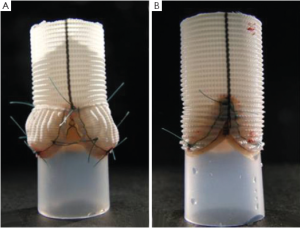
These in-vitro experiments were conducted by first creating two silicon roots with the same compliance, either with or without sinuses, containing the same biological stentless valve sutured inside following the remodeling technique (Figure 1, with permission). The two samples were then tested with simulated circulation to measure the pressure drop across the value, therefore calculating the effective orifice area at different cardiac outputs. The main finding of this simple experiment was that when the cardiac output was increased above 5 L/min the pressure drop significantly increased in the sample without sinuses of Valsalva. The results were identical when the portion of the aorta above the valve (the root) was replaced with either a straight Dacron graft (no sinuses) or with a Valsalva Dacron graft (with sinuses) (Figure 2, with permission): the pressure drop increased only in the sample without sinuses. This last finding appears to rule out compliance in having a role in modulating pressure drops across the valve. In fact, it was the shape, more than the compliance, which was responsible for the different findings (Figure 3, with permission). It was evident that in the presence of sinuses the valve was able to reach its full opening position; if the sinuses were absent complete opening was prevented.
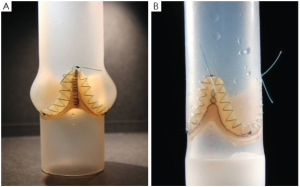
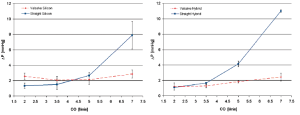
To further elucidate this aspect and to find a reasonable explanation for this different behavior, a second series of experiments was planned (8). In this series we aimed to ascertain if the pulsatility of flow played a role in generating fluid dynamics that could explain the differences in terms of the aortic valve pressure drop between the two different root configurations. We hypothesized that the interaction between the particular characteristics of pulsatile flow and the sinuses of Valsalva could be responsible for the superior hydrodynamic performance of the aortic valve.
The same two Dacron roots and valve models were prepared as described above: one with and one without sinuses (Figure 2, with permission). The hydrodynamic performance of the two assembled models was investigated in pulsatile and in laminar steady state flow regimes by using two dedicated test benches. Pressure drop and effective orifice area were measured at different flow rates. During the laminar steady state flow regime, it was evident that the hydrodynamic performance of the aortic valve was not markedly affected by the presence or absence of the sinuses.
Interestingly, from a comparison of the hydrodynamic test performed using a standard reference nozzle, in steady state flow regime, no difference was found in terms of pressure drop, either in the presence or absence of the sinuses, up to a cardiac output of 15 L/min (Figure 4). This observation shows the absence of any interaction between aortic root geometry and low-turbulence steady state flow regimes on hydrodynamic performance of the aortic valve. On the contrary, as shown in the previous study, the results acquired in pulsatile flow regimes showed significantly different behavior between the two root models. This implies that only in the presence of pulsatile flow do the sinuses of Valsalva play a role in modulating the pressure drop across the valve.
More specifically, when comparing the results of the two aortic root configurations operating under different flow regimes, it can be seen that the hydrodynamic performance of the aortic valve is markedly affected by the presence of pulsatile flow conditions. In this setting it appears that the interaction between aortic root geometry and pulsatile flow influences the opening of the aortic valve. Pulsatile flow is, by definition, turbulent flow; it is therefore likely that in the straight graft model and in the presence of pulsatile flow, the turbulence acts as a functional obstruction preventing complete opening of the aortic leaflets. On the contrary, in presence of appropriate space and architecture (represented by the sinuses of Valsalva), aortic leaflets are free to achieve complete valve opening with low-pressure drops, even in the presence of turbulent flow. This phenomenon could possibly be caused by the systolic vortices forming above the aortic valve. These vortices find a natural home in the sinuses expansion, which has an immediate effect both on the location and on the size of the vena contracta. The importance of flow turbulence in determining the effects described above was further confirmed by the finding that in steady conditions, when the flow was increased above 15 L/min (when it starts to become turbulent) (Figure 4) the same pressure drop across the valve started to build, but now in the absence of sinuses.
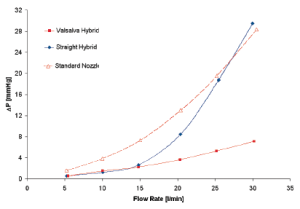
In conclusion, this series of experiments demonstrated that in the presence of turbulent flow (i.e., pulsatile flow), the particular shape of the aortic root and its sinuses are important in regulating proper opening of the aortic valve. In the absence of sinuses the turbulence of flow causes suboptimal opening of the valve with the consequent presence of a pressure drop and reduced effective orifice area.
How this finding might have implications for surgical technique is less clear. Although various techniques that do not provide sinuses reconstruction have proved to be equally effective in term of satisfactory valve function (9), it is already well established that in the absence of sinuses the valve closing mechanisms are impaired and reduced valve durability is to be expected (10). The observation that the presence of sinuses is also important in regulating valve opening, especially evident at higher cardiac output, contributes to the notion that in active, young and sportive patients the surgeon should strive to achieve an anatomical reconstruction of the aortic root.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Thubrikar MJ, Nolan SP, Aouad J, et al. Stress sharing between the sinus and leaflets of canine aortic valve. Ann Thorac Surg 1986;42:434-40.
- De Paulis R, De Matteis GM, Nardi P, et al. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg 2001;72:487-94.
- Robicsek F. Leonardo da Vinci and the sinuses of Valsalva. Ann Thorac Surg 1991;52:328-35.
- Morea M, De Paulis R. ‘Il buso. (the orifice). How much did Leonardo know of the aortic valve? J Cardiovasc Med (Hagerstown) 2007;8:399-403.
- Bellhouse BJ, Bellhouse FH. Mechanism of closure of the aortic valve. Nature 1968;217:86-7.
- Higashidate M, Tamiya K, Beppu T, et al. Regulation of the aortic valve opening. In vivo dynamic measurement of aortic valve orifice area. J Thorac Cardiovasc Surg 1995;110:496-503.
- Pisani G, Scaffa R, Ieropoli O, et al. Role of the sinuses of Valsalva on the opening of the aortic valve. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Salica A, Pisani G, Ieropoli O, et al. Straight versus Valsalva graft: importance of pulsatility and eddy currents during systole. An in vitro study. Eur J Cardio-Thor Surg 2012. [Epub ahead of print].
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622.
- Grande-Allen KJ, Cochran RP, Reinhall PG, et al. Re-creation of sinuses is important for sparing the aortic valve: a finite element study. J Thorac Cardiovasc Surg 2000;119:753-63.





