Anatomy of the aortic root: implications for valve-sparing surgery
Introduction
The aortic root connects the heart to the systemic circulation and is a highly sophisticated and complex structure. Each component of the aortic root - although simple in its macroscopic morphology - has an optimal macroscopic and microscopic structure and anatomical architecture (1) which contributes to the function of the aortic root as a whole: the intermittent, unidirectional channeling of large volumes of fluid, while maintaining laminar flow, minimal resistance and least possible tissue stress and damage, during varying haemodynamic conditions and demands (2-4). This well-coordinated dynamic behavior of all aortic root components has been shown to be of importance for specific flow characteristics, coronary perfusion and left ventricular function (5-8).
On the rare occasions when any aortic root component fails, it is the recognition of the complexity, scope and superiority of this structure—far better than any man-made replacement—that has led to the development of reparative, or ‘sparing’, surgical techniques that respect the functional and anatomical existence of the individual parts of the aortic root (9-12). For the communication and discussion of these techniques among surgeons a deep understanding of the anatomy of the aortic root and a generally accepted nomenclature is of fundamental importance (13-15).
Aortic root anatomy
The aortic root is an ensemble consisting of distinct entities: the aortic valve leaflets, the leaflet attachments, the sinuses of Valsalva, the interleaflet trigones, the sinotubular junction and the annulus (14,16-18) (Figure 1).
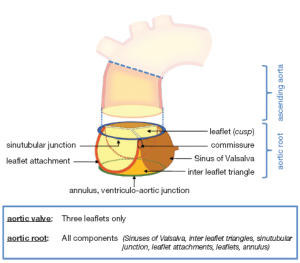
Aortic valve leaflets
The three leaflets form the aortic valve and provide its main sealing mechanism. Anatomically the valve leaflets can be divided into three parts:
- The free margin, with a thickened circular node (nodule of Arantius), which provides the coaptation area to the corresponding neighboring valve leaflets
- The “belly” of the leaflet
- The basal parts of the leaflet or leaflet attachments
The aortic valve leaflets form the hemodynamic junction and physical boundary between the left ventricle and the aorta. All the structures distal to the hemodynamic junction are subject to arterial pressures, whereas all the proximal parts are subjected to ventricular hemodynamics. The trileaflet design represents the optimal solution for low resistance valve opening (4). No other valve configuration can provide these characteristics, a fact prominently demonstrated in the setting of a bicuspid aortic valve, in which some kind of valve dysfunction or degree of stenosis always co-exists depending on the configuration (15).
Several surgical techniques have been developed for the correction of dysfunctional or misaligned leaflets leading primarily to aortic insufficiency. The required height and size of the leaflets to warrant competent valve function are primarily determined by the root size (19-22). These considerations seem to be a major determinant for the durability of aortic valve repair (23). Another important factor for the durability of the repair is adequate tissue quality, especially in the setting of bicuspid aortic valve disease.
Leaflet attachments
As the leaflet attachments insert in the wall of the aortic root they form a crown shaped, thick fibrous structure, often termed the “annulus”. This description is unfortunate as the word annulus implies a circular structure in contrast to the “crown” shape of the leaflet attachment (14). The points where the leaflet attachments run parallel - distally upstream towards the ascending aorta - are called the commissures.
Sinuses of Valsalva
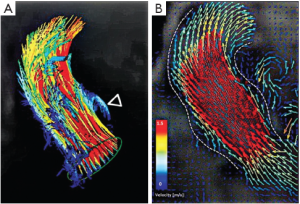
The three bulges of the aortic wall are named the sinuses of Valsalva, after the Italian anatomist Antonio Valsalva. Two of the three sinuses host the origin of the coronary arteries and the sinuses are termed accordingly the left, right and non-coronary sinus. They are limited proximally by the attachments of the valve leaflets and distally by the sinotubular junction. At their base, ventricular musculature is partly incorporated. The sinus wall itself is predominantly made up of aortic wall, although it is thinner than the native aorta (5,24,25). The precise function of the sinuses of Valsalva is unclear. There is evidence that the vortices created in the sinuses lead to stress reduction on the aortic leaflets and support coronary flow (4,5,24). In valve sparing aortic valve surgery, maintenance or recreation of the sinuses has been shown to effectively recreate the vortices in the sinuses and may be beneficial in terms of normal leaflet movement and valve durability (5,24-26) (Figure 2). However, aortic root reimplantation without recreation of the aortic sinuses has not been shown to have deleterious effects on valve durability despite abnormal leaflet motion (25). In general, there is a need for aortic root replacement material that imitates the compliant characteristics of the normal aortic root. This would allow for an ideal, and low stress, movement of the leaflets and could be a future area of interest and research not only for prosthetic material design but also for tissue engineering.
The interleaflet triangles
Under each commissure lies one of the three interleaflet triangles. Although histologically they consist of thinned aortic wall, hemodynamically they are extensions of the ventricular outflow tract and reach the level of the sinotubular junction in the area of the commissures.
The triangle between the right- and non-coronary sinuses faces the right atrium. It is in direct continuity with the membranous septum proximally which contains the His bundle. This area is of special importance during aortic valve procedures, as injury here can lead to temporary or permanent conduction abnormalities, which may require the implantation of a permanent pacemaker. Under the left and non-coronary triangle, the aorto-mitral curtain leads to the anterior mitral valve leaflet.
Sinotubular junction
The distal part of the sinuses toward the ascending aorta together with the commissures form a tubular structure called the “sinotubular junction” which separates the aortic root from the ascending aorta (Figure 1) (14). In some cases dilatation of the sinotubular junction is the cause of central aortic insufficiency and replacement of the ascending aorta with a short tubular graft can restore valve competence.
The “annulus”
Although the word annulus implies a circular structure, no distinct histological entity or anatomical boundary fits this description. The circumference defined by the nadirs of the semi-lunar leaflet attachments is difficult to define as the annulus, because there is no real, anatomically or histologically distinct, circular structure. The term ‘ventriculo-arterial junction’, as a definition of the “annulus”, is rather ambiguous as the ‘anatomical ventriculo-arterial junction’ represents the junction between the left ventricular myocardium and the arterial structure of the aorta. On the contrary, the ‘hemodynamic ventriculo-arterial junction’ is represented by the coronet shaped leaflet insertion, and defines the separation level of ventricular and arterial hemodynamics. From a strictly anatomic point of view, the ‘anatomic/histologic ventriculo-arterial’ as well as the ‘hemodynamic ventriculo-arterial’ junction lie somewhat more distally to the ‘annulus’ (16-18) and define the area of interest less precisely.
Despite the absence of any anatomically or histologically distinct circular structure the popularity of the term ‘annulus’ probably stems from the fact that this is the area of the smallest diameter in the blood path between the left ventricle and the aorta and determines the fitting position of prosthetic valve sizers and, therefore, the size of the prosthetic valve to be implanted. In addition to this, the use of this definition gives a good impression of the operative technique in use, such as the positioning of the prostheses ‘supra’ or ‘intra-annular’, as this is the level measured by echocardiographers as the ‘aortic valve annulus’ and is the area which defines the size of the prosthesis to be implanted during aortic valve replacement procedures. However prosthetic valves are inserted somewhat more proximally, more towards the level of the anatomic ventriculo-arterial junction, due to the placement of the sutures predominantly through the scalloped attachment of the excised leaflets, from the nadir of the sinus to midway up the commissures (27,28). In order to avoid any misunderstanding due to the numerous definition and terms employed, we have recently proposed the use of the term ‘annulus’ to describe the virtual, circular ring defined by the nadirs of the semi-lunar leaflet attachments (Figure 1) (14).
A successful aortic valve repair or sparing operation aims not only to correct the failing part of the aortic root, but also to restore the intra- and inter- component relationship of the aortic root elements to optimal dimensions and relations. The aortic annulus geometry and dimensions are major determinants of success and, more importantly, of the long term durability of aortic root repair. In cases with a dilated aortic annulus it can be successfully reduced using an internal or external ring (29-31). In the setting of valve sparing surgery, the David procedure also reduces the dimension of the annulus to fixed dimensions with excellent long term results (32).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Misfeld M, Sievers HH. Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci 2007;362:1421-36.
- Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair: from structure to function: Part II. Circulation 2004;109:1064-72.
- Yacoub MH, Kilner PJ, Birks EJ, et al. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg 1999;68:S37-43.
- Thubrikar MJ. The Aortic Valve. 1st ed. Boca Raton, Florida, US: CRC Press, Inc., 1981.
- Bellhouse BJ, Bellhouse FH. Mechanism of closure of the aortic valve. Nature 1968;217:86-7.
- Bellhouse BJ, Bellhouse FH, Reid KG. Fluid mechanics of the aortic root with application to coronary flow. Nature 1968;219:1059-61.
- Thubrikar M, Piepgrass WC, Bosher LP, et al. The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ Res 1980;47:792-800.
- Brewer RJ, Deck JD, Capati B, et al. The dynamic aortic root. Its role in aortic valve function. J Thorac Cardiovasc Surg 1976;72:413-7.
- Carr JA, Savage EB. Aortic valve repair for aortic insufficiency in adults: a contemporary review and comparison with replacement techniques. Eur J Cardiothorac Surg 2004;25:6-15.
- Gleason TG. Current perspective on aortic valve repair and valve-sparing aortic root replacement. Semin Thorac Cardiovasc Surg 2006;18:154-64.
- Miller DC. Valve-sparing aortic root replacement: current state of the art and where are we headed? Ann Thorac Surg 2007;83:S736-9;discussion S785-90.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007;22:473-9.
- Anderson RH. Demolishing the tower of babel. Eur J Cardiothorac Surg 2012;41:483-4.
- Sievers HH, Hemmer W, Beyersdorf F, et al. The everyday used nomenclature of the aortic root components: the tower of Babel? Eur J Cardiothorac Surg 2012;41:478-82.
- Sievers HH, Schmidtke C.A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33.
- Anderson RH. Clinical anatomy of the aortic root. Heart 2000;84:670-3.
- Anderson RH. The surgical anatomy of the aortic root. MMCTS 2007;2007:2527.
- Frater RW, Anderson RH. How can we logically describe the components of the arterial valves? J Heart Valve Dis 2010;19:438-40.
- Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010;38:400-6.
- Marom G, Haj-Ali R, Rosenfeld M, et al. Aortic root numeric model: Correlation between intraoperative effective height and diastolic coaptation. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Schäfers HJ, Schmied W, Marom G, et al. Cusp height in aortic valves. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Marom G, Haj-Ali R, Rosenfeld M, et al. Aortic root numeric model: Annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85.
- Schmidtke C, Sievers HH, Frydrychowicz A, et al. First clinical results with the new sinus prosthesis used for valve-sparing aortic root replacement. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Leyh RG, Schmidtke C, Sievers HH, et al. Opening and closing characteristics of the aortic valve after different types of valve-preserving surgery. Circulation 1999;100:2153-60.
- De Paulis R, De Matteis GM, Nardi P, et al. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg 2001;72:487-94.
- Sutton JP 3rd, Ho SY, Anderson RH. The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 1995;59:419-27.
- Sievers HH. Prosthetic aortic valve replacement. J Thorac Cardiovasc Surg 2005;129:961-5.
- Lansac E, Di Centa I, Raoux F, et al. An expansible aortic ring for a physiological approach to conservative aortic valve surgery. J Thorac Cardiovasc Surg 2009;138:718-24.
- Scharfschwerdt M, Pawlik M, Sievers HH, et al. In vitro investigation of aortic valve annuloplasty using prosthetic ring devices. Eur J Cardiothorac Surg 2011;40:1127-30.
- Rankin JS, Conger JL, Tuzun E, et al. In vivo testing of an intra-annular aortic valve annuloplasty ring in a chronic calf model. Eur J Cardiothorac Surg 2012;42:149-54.
- David TE, Feindel CM, Webb GD, et al. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg 2006;132:347-54.





