The role of echocardiography in aortic valve repair
Introduction
Echocardiography is the imaging method of choice for evaluating aortic valve repair for aortic regurgitation (AR). This article will discuss the role of echocardiography in the assessment of the severity, hemodynamics and mechanism(s) of AR, along with its role in the perioperative assessment of aortic valve repair.
Echocardiographic examination of the aortic valve and root
The aortic valve and root can be evaluated by transthoracic (TTE) or transesophageal echocardiography (TEE). Although TTE is useful in evaluating the severity of AR, TEE is more useful for assessing the mechanism of AR because of superior imaging quality. Alternatively, the aortic root can be assessed with cardiac magnetic resonance and multidetector computed tomography.
Because of the oblique orientation of the aortic valve, a multiplanar assessment of the valve is helpful in order to correctly align the valve plane with the ultrasonic beam. With TTE, this requires imaging from multiple echo windows, the preferred views being the parasternal long-axis and short-axis views. With TEE, one should combine the mid-esophageal aortic valve long-axis and short-axis views, the transgastric, and the deep transgastric long-axis views to obtain a complete assessment of aortic valve morphology and analysis of the mechanism of AR.
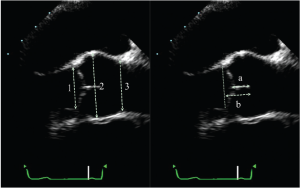
The aortic valve analysis should be performed in a systematic fashion. Imaging should start with long axis views, either parasternal or mid-esophageal. The long-axis view (obtained by TEE by rotating the multiplane angle to about 120°), permits visualization of the left ventricular outflow tract (LVOT), the aortic annulus, the aortic cusps, the sinuses of Valsalva, the sino-tubular junction (STJ) and the first centimeters of the tubular aorta (Figure 1). These measurements are performed in end-diastole, with the calipers placed at inner edges of the different structures. For the ascending aorta, the measurement should be made ±1 cm beyond the STJ. At end-diastole, the cusp coaptation can also be measured. Both the length of apposition (from the base to the tip of coaptation) and the coaptation height (analogous to the effective height measured intraoperatively with a caliper from the aortic annulus to the tip of coaptation) should be measured (Figure 1). It should be noted that from both the TTE parasternal view and the TEE mid-esophageal view, only the coaptation between the right coronary cusp anteriorly and either the non- or left coronary cusp (depending on probe rotation) posteriorly can be measured. Clockwise rotation with TEE identifies coaptation between the right coronary and the non-coronary cusps, whereas counterclockwise rotation permits evaluation of coaptation between right and left coronary cusps. Normal values for length of apposition is >4-5 mm and for coaptation height is 4-10 mm (which normally projects into the middle of the sinuses of Valsalva). In this view, color-flow imaging enables not only the analysis of the origin, but most importantly, the orientation of the regurgitant jet.
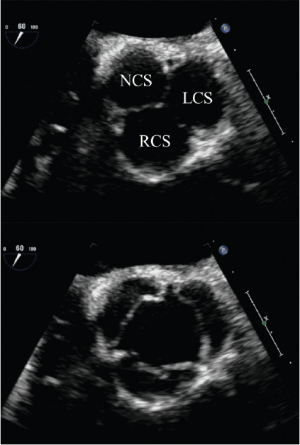
Short-axis TEE views are used for the analysis of both the symmetry of the cusps (Figure 2) and the origin of a regurgitant jet by color flow Doppler. For planning aortic valve repair, it is important to determine if regurgitation originates centrally, at a commissure, along the coaptation line or in the middle of one cusp. Bicuspid valves have important implications for the surgical strategy. Echocardiography is used to identify the presence and number of median raphes, the overall cuspal geometry, the presence of a cuspal prolapse, as well as the extent of possible cusp restriction and/or calcifications.
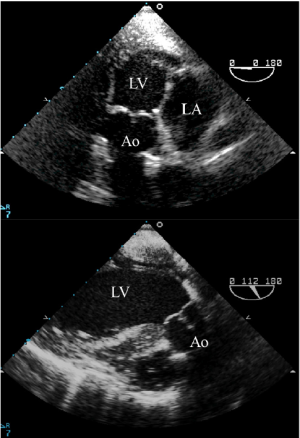
Both the TTE apical 3- or 5-chamber view and the TEE transgastric long-axis and the deep transgastric long-axis views also enable visualization of the aortic root and permit assessment of AR. With TEE, rotation of the transducer from 0 to 160 degrees may be required depending on the heart’s anatomical position (Figure 3). However, as the aortic valve is in the far field, a detailed anatomic assessment is often difficult and the severity of the jet may be underestimated.
Echocardiographic assessment of AR severity
There are several methods to evaluate the severity of AR. As none of them are 100% accurate, the assessment of AR severity should use all the information collected during the examination in an integrative and comprehensive way.
Color flow Doppler
Color flow Doppler allows for the semi-quantitative assessment of AR severity (1). Although the AR jet can be readily visualized in almost any imaging windows, parasternal (TTE) or mid-esophageal (TEE) views are usually preferred over the respective apical or transgastric views because of better axial resolution.
The color jet area and length are unsatisfactory measures of the severity of AR because of their load-dependence and are not recommended. They are nonetheless helpful in the identification of the mechanisms of AR, for example, eccentric jets being often associated with aortic cusp prolapse or perforation.
The diameter and the cross-sectional area of the color flow jet at its origin are better indices of AR severity. The maximum color jet diameter (width) is measured in diastole immediately below the aortic valve (at the junction of the LVOT and aortic annulus) in the parasternal long-axis view. The jet width is reasonably proportional to the size of the regurgitant orifice. Yet, if the orifice is irregular, as in bicuspid valves, the color jet width is less related to the degree of regurgitation. The accuracy of this method can be improved by dividing the jet width by the LVOT diameter. The cross-sectional areas of the jet from the parasternal short-axis view and its ratio to the LVOT area are also indicators of AR severity. Although these measurements suffer from a high inter-observer variability, a jet width ratio >65% is a strong argument for severe AR (1).
Vena contracta width
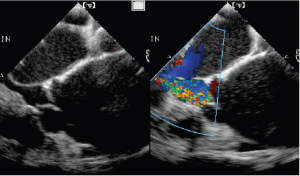
For AR, imaging of the vena contracta is best obtained from parasternal or mid-esophageal long-axis view (Figure 4). To properly identify the vena contracta, the three components of the regurgitant jet (the proximal convergence zone, the vena contracta and the jet area) must be visualized. The vena contracta represents the smallest flow diameter at the level of the aortic valve in the LVOT, immediately below the flow convergence region (2,3). A vena contracta width of <3 mm indicates mild AR, whereas a width >6 mm indicates severe AR. The concept of vena contracta is based on the assumption that the regurgitant orifice is circular. Unfortunately, in many instances (and particularly in patients with bicuspid valves), the orifice is elliptical or irregular. 3D color Doppler echo has been shown to be a useful tool in the visualization of the actual shape of the regurgitant orifice and can be used to measure the vena contracta. With 3D echo, an effective regurgitant orifice area (EROA) <20 mm2 and EROA >60 mm2 have been proposed to define mild AR and severe AR, respectively (4). However, these thresholds need to be confirmed in further studies.
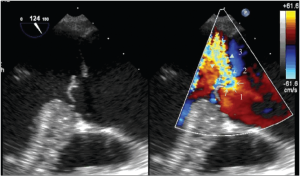
Mid-esophageal TEE grey scale (left panel) and color flow (right panel) long-axis views showing the 3 different components of an aortic regurgitant jet: the flow convergence zone (arrows), the vena contracta (arrow heads) and the jet width (triangles)
Flow convergence (PISA method)
Imaging of the flow convergence zone can be performed with TTE from the apical 3- or 5-chamber, the parasternal long-axis or the upper right parasternal views. With TEE, the mid-esophageal long-axis or deep transgastric views are used. The PISA calculation is illustrated in Figure 5. This method provides reliable estimates of regurgitant volume (RV) and EROA, except in patients with obtuse flow convergence angles (e.g., due to aneurysmal dilation of the ascending aorta) or with a confined flow convergence zone (e.g., due to commissural leak) (5). AR is classified as mild if the EROA <15 mm2 and RV <30 mL, moderate if the EROA is 15-30 mm2 and RV is 30-60 mL, and severe if the EROA ≥30 mm2 and/or RV ≥60 mL (6,7).
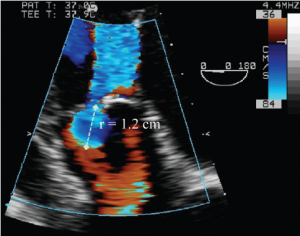
Deep transgastric long-axis TEE color flow view of the flow convergence zone of AR. To calculate the flow rate across the regurgitant orifice, the aliasing velocity was decreased to 36 cm/s (velocity at the blue-red border), which increased the radius of flow convergence (r). This is used to calculate regurgitant flow (= 2 × π × r2 × Valiasing)
Pulsed Doppler volumetric method
Pulsed wave Doppler can be used to calculate AR volume, which is the difference between left and right ventricular stroke volumes. However, this approach is time consuming and impractical for intraoperative use.
Diastolic flow reversal in the descending aorta
AR produces diastolic flow reversal in the aorta. This flow reversal is best imaged in the proximal descending aorta with pulsed Doppler. With mild AR, brief, early diastolic flow reversal occurs. As the severity of AR increases, the duration and the velocity of the reversal flow increases. With severe AR, holodiastolic flow reversal occurs (with end-diastolic velocity exceeding 20 cm/s, measured at peak R wave) (8).
Continuous-wave Doppler of the AR jet
CW Doppler of the AR jet reflects the pressure difference between the aorta and the left ventricle during diastole. The severity of regurgitation can be measured using the rate of deceleration of the diastolic regurgitant jet and the derived pressure-half time (Figure 6). As the severity of AR increases, aortic diastolic pressure decreases and LV end-diastolic pressure increases, shortening the pressure half-time. A pressure half-time <200 ms is consistent with severe AR, whereas >500 ms suggests mild AR. However, it is important to note that the pressure half-time is also influenced by chamber compliance in addition to chamber pressures (9).

Echocardiographic assessment of the hemodynamic consequences of AR
Chronic volume overload of the left ventricle due to severe AR may lead to progressive LV dilatation and irreversible damage. In asymptomatic patients with severe AR, surgery is indicated when LV ejection fraction is ≤50% and/or when LV end-systolic diameter is >50 mm or >25 mm/m2.
Echocardiographic assessment of the mechanisms of AR and prediction of reparability
Functional anatomy of AR
Aortic cusp and root lesions are categorized according to established surgical criteria (10). Briefly, the mechanism of AR is classified as Type 1: dilatation of the aortic root; Type 2: excess cusp motion (including cusp prolapses and free edge fenestrations) and good cusp tissue quality; and Type 3: poor cusp tissue quality (including cusp retraction, extensive cusp calcifications and/or endocarditis). Cusp prolapses can be further categorized in 3 groups: cusp flail (eversion of the cusp into the left ventricular outflow tract), partial or distal cusp prolapse, and whole cusp prolapse.
Evaluation of the mechanisms of AR by echocardiography follows the same principles (11):
- Type I dysfunction is identified as dilatation of any components of the aortic root, including the aortic annulus, the sinuses of Valsalva and the sino-tubular junction (Figure 7). Isolated dilatation of the ascending aorta not involving the aortic root does not cause AR.
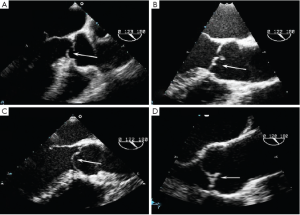
- Type 2 dysfunction is due to either cusp prolapse or fenestration and produces an eccentric AR jet. Cusp prolapse is diagnosed when the free edge of one or more aortic cusps overrides the plane of the aortic annulus. Three subtypes of cusp prolapse may be identified on echo: cusp flail, whole cusp and partial cusp prolapse. Cusp flail is complete eversion of a cusp into the LVOT (Figure 8A). Partial cusp prolapse is the distal portion of a cusp prolapsing into the LVOT, which is usually associated with bending of the cusp body seen on long-axis views (Figure 8B). In short-axis views, this leaflet bending may produce the appearance of a small circular or oval structure near the cusp free edge. Whole cusp prolapse is mostly seen in bicuspid valves and is diagnosed when the free edge of the cusp overrides the plane of the aortic annulus and its entire body billows into the LVOT (Figure 8C). Fenestration of the free edge of a cusp is diagnosed when there is an eccentric AR jet, but no signs of cusp prolapse. Careful inspection of long-axis grey-scale and color Doppler images may reveal small defects near the free edge of the affected cusp (Figure 8D).
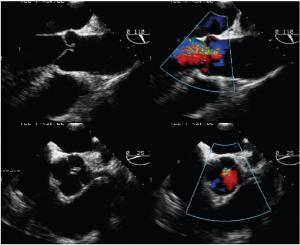
- Type 3 dysfunction is the presence of rigid, thickened and small aortic cusps relative to the aortic annulus, or infective endocarditis. It can also be seen in patients with quadricuspid aortic valves (Figure 9). Severely calcified valves are also included in this category.
Assessment of AV reparability
Unless severely calcified, most Type 1 and 2 AR lesions should be considered as potentially repairable with valve sparing surgery ± cusp repair. In moderately calcified valves, the localization of the calcifications should be taken into account. Aortic repair may be feasible if calcification is confined to the free margins, but not if calcification involves the body of the cusp. Type 3 lesions are not considered to be repairable (10).
Echocardiographic assessment of the results of AR repair
As with any valve repair procedure, one of the primary roles of TEE is the immediate evaluation of the quality of valve repair. The expected postoperative result is a flexible valve that opens well and is competent without any residual insufficiency.
We established the following algorithm for assessing aortic valve repair. This was based on a retrospective analysis of intraoperative echocardiographic data, incorporating functional and morphological features measured immediately after weaning from cardio-pulmonary bypass (12). Firstly, the level of cusp coaptation should occur above the aortic annulus. If cusp coaptation occurs below the aortic annulus, the risk of recurrent AR is >70% (12). Ideally, the height of coaptation should be greater than 9mm and reach the middle of the sinus of Valsalva. Secondly, the presence of any residual AR should be evaluated. If there is no residual AR and if coaptation is above the aortic annulus, recurrent AR is very unlikely. Finally, in patients with coaptation above the aortic annulus but who have residual AR, the length of cusp apposition should be measured. If this is <4 mm, it is associated with a 30-40% chance of recurrent severe AR in the follow-up (12).
Because of the potential for damage to the coronary arteries during aortic valve repair, regional wall motion abnormalities should be identified early.
Because aortic valve repair can reduce the valve orifice, mean and peak transvalvular gradients should be measured in the operating room to exclude significant stenosis. Mean and peak gradients in excess of 15 and 30 mmHg (respectively) are associated with an increased risk of developing aortic stenosis. However, these pressures may be under- or overestimated by echocardiography immediately post cardiopulmonary bypass depending on the patient’s flow conditions.
If the repair is unsatisfactory, a thorough echocardiographic assessment of the valve is necessary. The decision to revise or replace the valve depends on the underlying mechanism of AR, the quality of the valve tissue and on an assessment of patient factors such as age, comorbidities, and left ventricular function.The amount of residual AR by itself is not a satisfactory criterion for revision. The level at which coaptation occurs compared to the annulus and coaptation height are more predictive.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Perry GJ, Helmcke F, Nanda NC, et al. Evaluation of aortic insufficiency by Doppler colour flow mapping. J Am Coll Cardiol 1987;9:952-9.
- Tribouilloy CM, Enriquez-Sarano M, Bailey KR, et al. Assessment of severity of aortic regurgitation using the width of the vena contracta: A clinical color Doppler imaging study. Circulation 2000;102:558-64.
- Eren M, Eksik A, Gorgulu S, et al. Determination of vena contracta and its value in evaluating severity of aortic regurgitation. J Heart Valve Dis 2002;11:567-75.
- Fang L, Hsiung MC, Miller AP, et al. Assessment of aortic regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area: usefulness and validation. Echocardiography 2005;22:775-81.
- Pouleur AC, le Polain de Waroux JB, Goffinet C, et al. Accuracy of the flow convergence method for quantification of aortic regurgitation in patients with central versus eccentric jets. Am J Cardiol 2008;102:475-80.
- Tribouilloy CM, Enriquez-Sarano M, Fett SL, et al. Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol 1998;32:1032-9.
- Detaint D, Messika-Zeitoun D, Maalouf J, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging 2008;1:1-11.
- Tribouilloy C, Avinée P, Shen WF, et al. End diastolic flow velocity just beneath the aortic isthmus assessed by pulsed Doppler echocardiography: a new predictor of the aortic regurgitant fraction. Br Heart J 1991;65:37-40.
- Samstad SO, Hegrenaes L, Skjaerpe T, et al. Half time of the diastolic aortoventricular pressure difference by continuous wave Doppler ultrasound: a measure of the severity of AR? Br Heart J 1989;61:336-43.
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: Impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94.
- le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation. Accuracy, prediction of surgical repairability and outcome implications of transesophageal echocardiography. Circulation 2007;116:I264-9.
- le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair. Predictive value of intra-operative transesophageal echocardiography. JACC Cardiovasc Imaging 2009;2:931-9.





