Genes in thoracic aortic aneurysms/dissections - do they matter?
Introduction
Over the past decade, our knowledge of the genetic background of thoracic aortic aneurysms and dissections (TAAD) has expanded dramatically. This has not only led to a better understanding of the pathogenesis of the disease but also in risk stratification and medical guidance of patients and their families. Strategies for molecular genetic testing have reached a hinge point with the introduction of high throughput techniques based on Next Generation Sequencing (NGS) in routine diagnostics. It is therefore extremely important that clinicians in the field know the indications and limitations of molecular genetic testing. These will be reviewed in this manuscript.
Etiology and classification
The etiology of TAAD is complex and heterogeneous. Degenerative aortic disease related to classic cardiovascular risk factors such as smoking, arterial hypertension and hyperlipidemia are the main cause of TAAD in older patients. In younger patients with no risk factors, other causes, including genetic disease, should be considered. Genetic aneurysmal disease can be categorized in 3 main groups: (I) inherited syndromes predisposing to early onset TAAD (<5% of all TAAD) such as Marfan syndrome (MFS), Loeys-Dietz syndrome (LDS) and aneurysm-osteoarthritis syndrome (AOS) (1-3); (II) familial forms of TAAD (FTAAD -20% of all TAAD), including patients with confirmed disease in first-degree relatives and evidence for an autosomal dominant inheritance pattern; these patients may sometimes present with associated cardiovascular lesions such as bicuspid aortic valve (BAV), patent ductus arteriosus (PDA), or cerebrovascular disease (4-6); and (III) isolated or sporadic forms of TAAD including young subjects with no family history or features of syndromic forms. The latter two categories are the so-called non-syndromic forms of TAAD as opposed to the syndromic forms of the first category.
The differentiation between these subgroups and diagnostic work-up of an individual patient is primarily based on detailed clinical evaluation of the proband and family members (see below). Additional molecular genetic testing may be helpful and sometimes even required for confirmation of the specific diagnosis.
Table 1 provides an overview of syndromic and non-syndromic forms of TAAD with their corresponding genes and clinical features. Discriminative features are in bold.
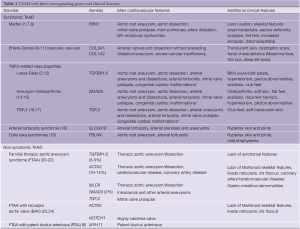
Full table
As can be appreciated from this list, substantial clinical overlap exists between these entities, which may hamper confirmation of the correct diagnosis.
Until very recently, strategies for genetic testing were limited as only one gene could be analyzed at a time and both the time required as well as the costs for screening of multiple genes were substantial. These limitations have now become redundant with the introduction of high-throughput Next Generation Sequencing based techniques. Correct interpretation of the results obtained by molecular genetic testing requires basic knowledge of these different entities - all the more since medical and surgical management may differ according to the underlying diagnosis.
Strategy for clinical and genetic evaluation
The absolute prerogative for further clinical/genetic investigations in TAAD patients is a correct diagnosis of the aneurysm itself, which should be based on careful measurement of the diameter of the aorta according to appropriate guidelines (25). The diameter obtained needs to be correlated to values in normal subjects matched for age, body surface area and gender (26). To correlate with normal values, nomograms can be used or z-scores can be calculated - the latter method being more convenient for reporting. Aortic dilatation is recognized if the z-score exceeds 2, corresponding to an observed value >1.96 Standard Deviations above the predicted value for age, gender, and body size. In children, growth needs to be taken into account and z-scores >3 have been suggested (27).
Further investigations will depend on the age and cardiovascular risk profile of the patient. Consideration of a genetic entity is especially useful in young subjects with no additional risk factors.
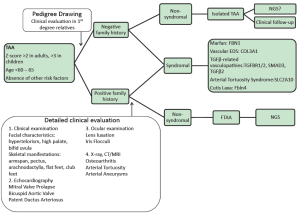
Detailed family history taking, including pedigree drawing and clinical assessment of first degree relatives is required to differentiate between familial and isolated cases. Next, careful multidisciplinary clinical evaluation of the proband is undertaken, which will help us in the identification of specific syndromes as reported in Table 1. If clinical findings point towards a specific genetic condition, focused mutation analysis can be undertaken - for example in patients presenting with a TAA in combination with lens luxation, Marfan syndrome is very likely and molecular genetics can be restricted to the FBN1 gene. From a cardiovascular perspective, extensive vascular disease such as aortic aneurysms at different locations and/or involvement of side branches makes a diagnosis of Marfan syndrome much less likely and in these cases TGFβ-associated disease should be excluded first. Figure 1 shows some examples of extended vascular disease in Loeys-Dietz and aneurysm-osteoarthritis syndrome patients. In many other cases, the diagnosis may be less straightforward and screening of a panel of genes may be more suitable. In our laboratory, we set up 2 NGS panels for TAA screening including 16 genes (Panel 1: FBN1, TGFBR1/2, SMAD3, TGFb2, ACTA2, COL3A1 and Panel 2: MYH11, MLCK, SLC2A10, NOTCH1, FBN2, ADAMTS10, FBLN4, FLNA, ELN). In familial cases of non-syndromic TAAD, exclusion of genetic defects in multiple known genes is required – in this setting NGS may be particularly useful.

A flow chart illustrating the diagnostic process is provided in Figure 2.
Genes and pathogenesis
In addition to its usefulness in a diagnostic setting, molecular genetics have been very useful in unravelling the complex pathogenesis of TAA formation.
One of the most inspiring findings over recent years was the observation of the involvement of the transforming growth factor β (TGFβ) pathway in several connective tissue disorders. The TGFβ superfamily consists of a number of cytokines that regulate diverse cellular functions, including proliferation, differentiation, and synthesis of a wide array of gene products.
The first connective tissue disorder linked to the TGFβ pathway was Marfan syndrome (MFS). The underlying pathogenesis of aneurysm formation in MFS was initially considered to be a consequence of inherent structural weakness of the tissues due to structurally abnormal fibrillin-1 fibres. Prospects for causal treatment were pessimistic in this view since this would require a means to alter the structural composition of inherently weak tissues.
Fortunately, recent developments have changed this insight and it is now recognised that fibrillin-1 fibres also play an important functional role in the complex TGFβ pathway. Sakai and co-workers demonstrated that fibrillin-1 was homologous with the family of latent TGFβ binding proteins (LTBPs), which serve to hold TGFβ in an inactive complex in various tissues, including the extracellular matrix (28). Researchers showed that fibrillin-1 can bind TGFβ and LTBPs (28-31).
Since the large latent complex binds TGFβ, abnormal fibrillin-1 fibres will lead to failed matrix sequestration of the latent TGFβ complex and hence increase amounts of active TGFβ, which is in turn at the basis of the pleiotropic manifestations in MFS (32). Indeed, increased TGFβ signalling has been demonstrated in aortic tissue samples and in mitral valve from patients with MFS.
These findings have opened new perspectives for treatment through inhibition of TGFβ-signalling. An initial experiment with TGFβ neutralizing antibodies in a mouse model for MFS showed a dramatic decrease in aortic root growth as well as restoration of aortic wall architecture (33). From studies in nephrology, it was documented that losartan, an angiotensin receptor blocking agent, inhibits TGFβ signalling. A trial with losartan in MFS mice showed significant rescue of aortic root aneurysm progression as well as aortic wall architecture compared to treatment with either placebo or propranolol (33). A subsequent small study in children with severe MFS showed similar promising results (34). Large-scale trials in MFS patients are currently underway (35).
Additional evidence for the involvement of the TGFβ pathway in aneurismal disease comes from the finding that mutations in several genes that encode different components of the pathway result in aneurysm conditions that have undeniable clinical overlap with MFS. Initially, these disorders were given names - the first one being the Loeys-Dietz syndrome (LDS), caused by mutations in the TGFBR1 and TGFBR2 genes. In 2011, mutations in the SMAD3 gene were identified in patients with a very similar phenotype but also presenting osteoarthritis, hence the name “aneurysm-osteoarthritis syndrome”. Soon thereafter a family with juvenile polyposis associated with aortopathy and mitral valve disease caused by SMAD4 mutations was reported (36) and finally, mutations in the TGFβ-2 ligand were very recently identified in several families displaying a very similar phenotype (17,37,38). As already mentioned, clinical differentiation between these entities may be hampered by significant overlap and additional genetic testing may be particularly helpful in these cases.
It is clear that these disorders are all part of a broad spectrum and it may be more convenient to group them under the term “TGFβ-related vasculopathies”.
Figure 3 provides a schematic and abbreviated overview of the TGFβ signalling pathway with indication of aneurismal diseases linked to it.
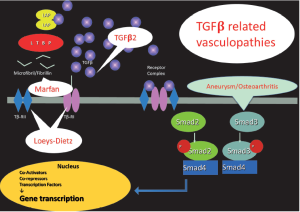
Increased TGFβ signalling has also been reported in human aortic specimens of patients with isolated TAAD and underlying ACTA2 or MYH11 mutations (39). The exact link is not yet fully understood but links between the cytoskeleton and many aspects of the TGFβ signalling pathway have been established, including trafficking and activity of TGFβ receptors and signalling effectors (40,41).
Gene-tailored follow-up and management in TAAD
A schematic overview of the medical management is provided in Table 2.
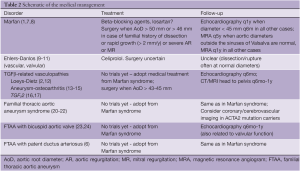
Full table
Imaging studies
Confirmation of the exact diagnosis in the proband facilitates the set-up of a personalized strategy for follow-up and treatment in the patient and their family. Since expression of the disease may be delayed, lifelong follow-up is required in all mutation-carriers, even if aortic diameters are normal upon repeated measurements. The frequency and modality of follow-up and treatment may differ according to the underlying diagnosis as summarized in Table 2. Importantly, clinical monitoring and follow-up with cardiovascular imaging is also warranted in family members of TAAD patients in whom no causal mutation was identified since familial clustering is observed in more than 20% of TAAD cases (42,43).
Echocardiography is the primary imaging tool for evaluation and follow-up of the diameters of the aortic root and ascending aorta. CT or MRI can be used in case of insufficient visualization of the ascending aorta by echocardiography. The imaging study should be repeated in all patients six months after the initial diagnosis to assess evolutionary changes. Further follow-up is guided by the diameter, evolution, underlying diagnosis and family history. Stable diameters <45 mm in patients with Marfan syndrome or isolated TAA and no family history for dissection require yearly follow-up. Bi-annual controls are recommended in all other cases.
Imaging of the entire aorta and side branches (“head-to-pelvis” study) should be performed at diagnosis in order to detect aneurysms and/or arterial tortuosity at other sites. Regular extensive vascular imaging from head to pelvis is recommended in patients with a TGFBR1/2, SMAD3 and TGFβ2 mutation and for rare diseases such as cutis laxa and arterial tortuosity syndrome (ATS). In vascular Ehlers-Danlos syndrome (EDS) where dissections often occur at normal diameters, the modality and frequency of vascular imaging is debatable. Evaluation for coronary artery and cerebrovascular disease can be considered in patients with an ACTA2 mutation (44).
Medical treatment
The initial medical approach of TAAD patients should include reduction of cardiovascular risk factors, such as blood pressure control, smoking cessation and optimization of the lipid profile. Central stimulating drugs, such as cocaine, amphetamine and derivatives are known triggers for aortic dissection and should therefore be avoided (45,46).
Medical treatment with a β-blocking agent in MFS reduces the progression of aortic dilatation in most patients through reduction of wall shear stress in the aorta and is used as standard therapy in MFS patients (47). The effect of losartan in reducing aortic root growth is unclear at the moment and results from ongoing trials need to be awaited prior to large-scale prescription. In patients with vascular EDS, reduction of fatal vascular events was observed with treatment with celiprolol, a β-blocker with β2 mimetic action (48). The possible role of medical treatment in other TAAD diseases is not well studied but pragmatically, treatment as for MFS is adopted.
Surgery
It is beyond doubt that elective surgical aortic root replacement leads to better survival in patients with genetic aortic disease. Modalities for surgical intervention are beyond the scope of this contribution and will be addressed elsewhere in this issue. We do want to spend some words on the timing of surgery taking the underlying diagnosis into account.
It has been demonstrated that the risk for dissection or rupture for thoracic aortic aneurysms of non-degenerative origin rises at lower diameters when compared to degenerative aortic disease. Accordingly, the threshold for surgery of the aortic root is lower than the conventional 55 mm. According to the ESC guidelines on Grown-up Congenital Heart Disease, the conventional surgical indication for MFS is an aortic diameter - measured at the sinuses of Valsalva - of 50 mm or more. This threshold is reduced to 46 mm in case of a positive family history of aortic dissection and in case of a rapid growth of the aorta (>2 mm/year). When there is a desire for pregnancy, aortic repair is recommended at 45 mm (49). In certain other syndromic and non-syndromic TAAD entities such as LDS or AOS, aortic dissection can appear at smaller diameters, which requires an adjusted treatment policy. Results of surgical intervention in LDS and AOS are good (3,50). Taking these data into account, the current guidelines of the American College of Cardiology recommend prophylactic surgery in the following cases (51,52): (I) in patients with a mutation in TGFBR1 or TGFBR2 (as well as patients with LDS as familial TAAD): when the diameter of the ascending aorta reaches 42 mm measured by echocardiography or 44-46 mm on CT or MR imaging; (II) in patients with familial TAAD and/or mutation in MYH11 or ACTA2: when the diameter of the ascending aorta measures between 45 and 50 mm; (III) in patients with familial TAAD with relatives with an aortic dissection: at minimal dilatation of the thoracic aorta (<50 mm); (IV) for all other TAAD patients: when the ascending aorta or aortic root reaches a diameter of 50 mm, in cases of rapid growth of the aorta (≥5 mm/y) and/or in the presence of severe aortic stenosis or regurgitation. Patients with a mutation in the MYLCK gene can have an aortic dissection at minimal diameters of the aorta, as indicated by the study of Wang et al. (53). Guidelines regarding the role of prophylactic surgery in this group of patients are lacking. In contrary to patients with TGFBR2 mutation, aortic dissection in patients with a TGFBR1 mutation would rather occur at larger diameters (>50 mm) (54). In view of these data, early referral for surgery may be questioned.
Conclusions
In the current era of improved availability of high throughput molecular genetic techniques, knowledge of the indications and limitations for these tests in daily clinical practice is increasingly important. In the case of TAAD, additional genetic testing may be helpful for confirmation of the correct diagnosis. Since follow-up and treatment of patients may be adapted according to the underlying condition, clinicians dealing with these patients should acquire this knowledge. Close collaboration between cardiovascular surgeons, cardiologists and clinical geneticists is strongly recommended in the care of these patients and families.
Acknowledgements
Disclosure: Julie De Backer is holder of a Senior Clinical Research Grant from the Flanders Funds for Scientific research, Anne De Paepe is holder of a Methusalem Grant from the Flanders Funds for Scientific Research.
References
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005;366:1965-76.
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788-98.
- van der Linde D, van de Laar IM, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol 2012;60:397-403.
- Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 2009;84:617-27.
- Regalado E, Medrek S, Tran-Fadulu V, et al. Autosomal dominant inheritance of a predisposition to thoracic aortic aneurysms and dissections and intracranial saccular aneurysms. Am J Med Genet A 2011;155A:2125-30.
- Khau Van Kien P, Wolf JE, Mathieu F, et al. Familial thoracic aortic aneurysm/dissection with patent ductus arteriosus: genetic arguments for a particular pathophysiological entity. Eur J Hum Genet 2004;12:173-80.
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85.
- Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991;352:337-9.
- Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000;342:673-80.
- Malfait F, Symoens S, De Backer J, et al. Three arginine to cysteine substitutions in the pro-alpha (I)-collagen chain cause Ehlers-Danlos syndrome with a propensity to arterial rupture in early adulthood. Hum Mutat 2007;28:387-95.
- Malfait F, Symoens S, Coucke P, et al. Total absence of the alpha2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J Med Genet 2006;43:e36.
- Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275-81.
- van de Laar IM, van der Linde D, Oei EH, et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet 2012;49:47-57.
- van der Linde D, van de Laar IM, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol 2012;60:397-403.
- van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 2011;43:121-6.
- Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 2012;44:922-7.
- Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 2012;44:916-21.
- Coucke PJ, Willaert A, Wessels MW, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet 2006;38:452-7.
- Renard M, Holm T, Veith R, et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet 2010;18:895-901.
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488-93.
- Milewicz DM, Grossfield J, Cao SN, et al. A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest 1995;95:2373-8.
- Hasham SN, Guo DC, Milewicz DM. Genetic basis of thoracic aortic aneurysms and dissections. Curr Opin Cardiol 2002;17:677-83.
- Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270-4.
- McKellar SH, Tester DJ, Yagubyan M, et al. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2007;134:290-6.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63.
- Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol 2012;110:1189-94.
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85.
- Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 2003;278:2750-7.
- Dallas SL, Keene DR, Bruder SP, et al. Role of the latent transforming growth factor beta binding protein 1 in fibrillin-containing microfibrils in bone cells in vitro and in vivo. J Bone Miner Res 2000;15:68-81.
- Dallas SL, Miyazono K, Skerry TM, et al. Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J Cell Biol 1995;131:539-49.
- Saharinen J, Hyytiäinen M, Taipale J, et al. Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev 1999;10:99-117.
- Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003;33:407-11.
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117-21.
- Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008;358:2787-95.
- Lacro RV, Dietz HC, Wruck LM, et al. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J 2007;154:624-31.
- Andrabi S, Bekheirnia MR, Robbins-Furman P, et al. SMAD4 mutation segregating in a family with juvenile polyposis, aortopathy, and mitral valve dysfunction. Am J Med Genet A 2011;155A:1165-9.
- Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 2012;44:922-7.
- Renard M, Callewaert B, Malfait F, et al. Thoracic aortic-aneurysm and dissection in association with significant mitral valve disease caused by mutations in TGFB2. Int J Cardiol 2012. pii: S0167-5273(12)01135-7.
- Renard M, Callewaert B, Baetens M, et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ signaling in FTAAD. Int J Cardiol 2011. [Epub ahead of print].
- Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development 2009;136:3699-714.
- Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308-16.
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999;134:361-7.
- Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 2006;82:1400-5.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129.
- Westover AN, Nakonezny PA. Aortic dissection in young adults who abuse amphetamines. Am Heart J 2010;160:315-21.
- Daniel JC, Huynh TT, Zhou W, et al. Acute aortic dissection associated with use of cocaine. J Vasc Surg 2007;46:427-33.
- Shores J, Berger KR, Murphy EA, et al. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med 1994;330:1335-41.
- Ong KT, Perdu J, De Backer J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 2010;376:1476-84.
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57.
- Williams JA, Loeys BL, Nwakanma LU, et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg 2007;83:S757-63; discussion S785-90.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86.
- Milewicz DM, Regalado E. Thoracic Aortic Aneurysms and Aortic Dissections. In: Pagon RA, Bird TD, Dolan CR, et al. eds. GeneReviews. Seattle WA: University of Washington, Seattle, 1993.
- Wang L, Guo DC, Cao J, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 2010;87:701-7.
- Tran-Fadulu V, Pannu H, Kim DH, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet 2009;46:607-13.





