Barrett’s esophagus: best practices for treatment and post-treatment surveillance
Introduction
Barrett’s esophagus (BE) is the only known precursor lesion of esophageal adenocarcinoma (EAC). Endoscopically, BE appears as an area of salmon-colored mucosa in the distal esophagus. However, the endoscopic appearance alone is not sufficient for a diagnosis of BE, and histological specimens showing intestinal metaplasia (IM) in the esophagus are required in order to meet the current definition of BE (1).
BE is primarily a disease of the Western world, although it is increasingly described in Asian countries (2). The prevalence of BE in patients undergoing endoscopy for any indication has been estimated to be at 1–2%, and this increases to 5–15% in patients that have GERD symptoms (3). Risk factors for BE include older age, male sex, Caucasian race, GERD symptoms (especially starting at a young age), central abdominal obesity, and possibly tobacco smoking (4).
It is believed that most—if not all—EAC develops from areas of BE. Studies have estimated that patients with BE have a significantly increased risk (30- to 125-fold) of developing EAC compared to patients without BE (4). However, the absolute risk of EAC in patients with non-dysplastic BE is low, at 0.1–0.5% per year, with most recent studies suggesting the incidence is on the lower end of that range (5-7). The risk for progression to EAC is higher in patients with dysplasia, with an annual risk of approximately 1% in patients with low grade dysplasia (LGD), and 7–19% in patients with high grade dysplasia (HGD) (8-10).
EAC is usually identified in patients with BE that was either previously undiagnosed or who have not been in surveillance. Diagnosis under these circumstances is typically at an advanced stage and carries a poor prognosis, with a five-year survival of approximately 17% (8). Therefore, there has been a significant focus on prevention and early detection, primarily by screening for BE, surveillance in BE, and treatment of dysplastic BE. The advances in the endoscopic management of Barrett’s dysplasia and early EAC have eliminated the need for radical esophageal surgery in many patients. In this review, we will discuss screening, surveillance, advanced imaging, chemoprevention, and endoscopic eradication therapy (EET) of BE, with a focus on ablative techniques and post-treatment surveillance.
Screening for BE
Mass screening for BE or EAC at a population-wide level is not cost-effective (11). While not supported by high level evidence, screening in high-risk patients is advised based on expert opinion. For example, guidelines published in 2016 from the American College of Gastroenterology recommend endoscopic screening for BE in men with chronic (>5 years) and/or frequent (weekly or more) GERD symptoms and two or more risk factors for BE or EAC, including age >50, Caucasian race, central obesity, history of tobacco smoking, and a family history (first-degree relative) of BE or EAC (12).
While upper endoscopy remains the gold standard for BE screening, it is associated with considerable costs related to endoscopy, histopathology examination, and sedation. Consequently, less invasive screening tools, which can be performed without the need for sedation, have been proposed, including transnasal endoscopy (TNE) and esophageal capsule cytology. TNE can be performed in an office setting without sedation. Studies have demonstrated that TNE provides acceptable image quality and biopsies that, while small in size, are still sufficient for acceptable histologic analysis (13,14). TNE is also generally preferred by patients and is associated with less patient anxiety than conventional EGD. However, TNE has not been adopted by primary care providers due to concerns about patient tolerance of an unsedated procedure and uncertainties regarding the safety, time, training, and facilities required for this procedure. For gastroenterologists and other endoscopists, there is an additional financial disincentive for performing an office-based procedure with lower reimbursement than standard upper endoscopy (15).
Esophageal capsule cytology (Cytosponge) is a non-invasive, non-endoscopic method of screening for BE that was first described in 2007 (16). The device is a sponge within a capsule covered in a gelatin layer which dissolves after swallowing, after which the sponge is released. A string attached to the device is used to pull it from the stomach, across the gastroesophageal junction and out through the mouth, brushing along the esophageal mucosa and capturing cells for analysis. Immunohistochemistry is then performed to analyze for markers such as trefoil factor 3, which can differentiate the cells of BE from other columnar cells found in the normal gastric cardia and upper airway (16). In a prospective cohort study of 504 patients with GERD symptoms treated with either an H2 blocker or PPI in the United Kingdom, the sensitivity and specificity of Cytosponge compared with EGD was 73.3% and 93.8%, respectively, for BE ≥1 cm (17). A multi-center case-control study from the United Kingdom published in 2015 comparing Cytosponge to upper endoscopy with biopsy in 647 patients and 463 controls showed that Cytosponge had an overall sensitivity and specificity of 79.9% and 92.4%, respectively (18). Further validation of this technique and the tested biomarkers in other practice settings is required before wider adoption can be recommended.
Screening for BE has several limitations. Risk-based screening may miss a considerable proportion of BE, especially those who are at high risk of EAC. For example, it has been demonstrated that at least 40% of patients who are ultimately diagnosed with EAC reported no history of typical GERD symptoms (19,20). Additionally, a systematic review of 12 studies published between 1988 and 1999 has shown that only 4.7% of patients undergoing surgery for EAC carried a prior diagnosis of BE (21). These findings show that there is still much work to be done in order to optimize screening strategies for BE.
Surveillance for neoplasia in BE
Endoscopic surveillance in patients with known BE has been advocated by multiple professional societies, with the goal of detecting dysplasia or early EAC so that curative treatment can be offered prior to the development of advanced EAC. While evidence from randomized trials is lacking (and will be very difficult to attain given logistical challenges), observational studies have shown improved outcomes in patients with EAC who had been undergoing surveillance for BE as opposed to those without Barrett’s surveillance. Some of the best evidence from this comes from a cohort study of nearly 30,000 patients with BE from the national VA database, which showed that patients that were diagnosed with EAC as part of a BE surveillance program were more likely to be diagnosed with early stage disease, had improved survival, and lower cancer-related mortality compared with patients diagnosed with EAC who were not part of a BE surveillance program (22).
The principles of endoscopic surveillance in BE include careful inspection of the Barrett’s mucosa with high-definition white light endoscopy (HDWLE). Many experts additionally advocate the routine use of electronic chromoendoscopy [such as narrow band imaging (NBI)]. Adequate time should be spent inspecting the Barrett’s epithelium; a study published in 2012 showed a significant correlation between the Barrett’s inspection time and the yield of HGD or EAC, with significantly higher detection with an inspection time of ≥1 minute per centimeter of BE (23). Nodular lesions or visible abnormalities within Barrett’s epithelium should be endoscopically resected or biopsied and placed in separate containers. Systematic random biopsies are then obtained from the Barrett’s mucosa according to the Seattle protocol (24): four-quadrant biopsies every 2 cm (or every 1 cm in patients with known or suspected dysplasia). However, there has been a recent push from several experts to transition from random biopsies to imaging-directed targeted biopsies, though random biopsies remains the standard of care at this time (25).
Surveillance intervals and management options vary according to the presence and grade of dysplasia. For non-dysplastic BE (NDBE), most societal guidelines recommend a repeat surveillance endoscopy in 3–5 years. For dysplastic BE of any grade, review by two pathologists (at least one of whom is an expert GI pathologist) is recommended due to the high degree of inter-observer variability among pathologists in diagnosing and classifying dysplasia in BE. The importance of confirmation of the degree of dysplasia cannot be overstated. In a large retrospective cohort from the Netherlands of 293 patients with LGD, 73% of the cases previously labeled as LGD were downgraded to NDBE or indefinite for dysplasia and the other 27% were confirmed as LGD after review by an expert panel of pathologists. In patients with confirmed LGD, the risk of progression to HGD/EAC was 9.1% annually, compared with 0.6% in patients with NDBE. While close surveillance has been advocated for LGD in the past and the optimal management of LGD in BE remains controversial, most recent data and recommendations favor endoscopic ablative therapy for confirmed LGD. For patients with LGD who opt for surveillance instead, endoscopy should be performed annually. Patients with HGD should be offered EET with either ablation and/or endoscopic resection (ER) (12).
While surveillance of BE appears to be beneficial, the current surveillance strategies are inefficient and include risks of endoscopy and sedation, the possibility of missed lesions, and complications from curative treatment. Therefore, it is important that future research focus on improving the value of BE surveillance by optimizing effectiveness and efficiency. One area being studied for this purpose is the use of advanced imaging technologies.
Advanced imaging
The systematic four-quadrant biopsies Seattle protocol is relatively inefficient and has a low diagnostic yield for neoplasia (26). Consequently, there has been interest in studying the use of endoscopic advanced imaging techniques in order to improve the efficiency and diagnostic yield, and reduce the cost of BE surveillance. In 2012, The American Society for Gastrointestinal Endoscopy (ASGE) released minimum criteria under their Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) initiative in order to determine whether an advanced imaging technology with targeted biopsies could replace the current standard of care of random four-quadrant biopsies (27). It was proposed that, in order to eliminate the need for random biopsies in BE surveillance, an imaging technology with targeted biopsies should have a per-patient sensitivity of ≥90% and a negative predictive value (NPV) of ≥98% for detecting HGD or early EAC compared with the current standard protocol (high definition white-light endoscopy with targeted and random 4-quadrant biopsies every 2 cm). Additionally, the specificity should be sufficiently high (≥80%) to allow for a decrease in the number of biopsies (compared with random biopsies). In 2016, a systematic review and meta-analysis was conducted by the ASGE technology committee and reported that acetic acid chromoendoscopy, NBI, and endoscope-based confocal laser endomicroscopy (eCLE) met the proposed PIVI thresholds (Table 1) (28).
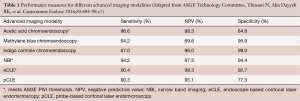
Full table
Dye-based chromoendoscopy involves the use of dyes or chemicals that are applied onto the mucosa to enhance the visualization of the subtle mucosal and microvascular patterns. The three best studied dyes/chemicals are methylene blue, indigo carmine, and acetic acid. Early studies of methylene blue chromoendoscopy for BE concluded that it improves detection of IM and dysplasia in BE (29). However, subsequent studies were equivocal, and a meta-analysis that included studies from 2000–2008 concluded that methylene blue chromoendoscopy was not superior to standard 4-quadrant biopsies in detecting IM or dysplasia in patients with BE (30). In a prospective multicenter cohort study of 56 BE patients, targeted biopsies using magnification endoscopy in combination with indigo carmine chromoendoscopy showed a sensitivity of 67–83% for detecting HGD depending on the specific pattern of mucosal abnormality (31). Acetic acid is a colorless agent that reacts with and enhances the mucosal surface pattern, and has been shown to accurately distinguish between normal esophagus, BE, and neoplasia. In the ASGE technology group meta-analysis, a subgroup analysis of studies focusing on acetic acid chromoendoscopy demonstrated an overall sensitivity, NPV, and specificity of 96.6%, 98.3%, and 84.6%, respectively for detecting HGD/EAC (28). Despite its documented usefulness, particularly with acetic acid, dye-based chromoendoscopy has not gained widespread clinical use, mainly due to the perception that it is tedious, inhomogeneous, and time-consuming, as well as lack of separate reimbursement.
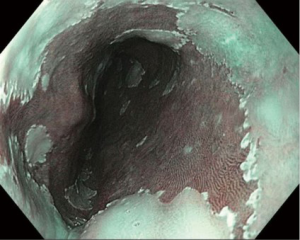
In contrast, electronic/optical chromoendoscopy is much more commonly used. These technologies use filters within the endoscope to emit light at certain wavelengths and/or specialized computer processing technology to enhance the visualization of the mucosal surface. These technologies are considerably easier to use than dye-based chromoendoscopy and can be turned on and off by pushing a button on the handle of the endoscope. The three major forms of electronic chromoendoscopy which are commercially available are narrow-band imaging (NBI; Olympus, Center Valley, PA, USA), I-Scan (Pentax Medical, Montvale, NJ, USA), and Fujinon intelligent color enhancement (FICE; Fujinon, Inc., Wayne, NJ, USA). NBI (Figure 1) is generally the most widely available system, and subsequently the one most studied. NBI was shown to have >90% sensitivity and specificity for the detection of high grade dysplasia or EAC in two meta-analyses (28,32).
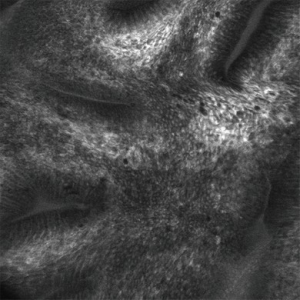
Confocal laser endomicroscopy (CLE) allows for in vivo microscopic level (1,000× magnification) evaluation of the epithelium (Figure 2). There are two forms of CLE: one is built in to the endoscope (eCLE) and another uses a probe that can be inserted through the working channel of an endoscope (pCLE). Both require the administration of a fluorescent agent, typically intravenous fluorescein. In a multi-center, international, randomized controlled trial published in 2014, it was shown that eCLE was able to significantly increase the diagnostic yield for HGD and EAC compared to the current standard of care (high definition white light endoscopy with random four-quadrant biopsies) (33). In a meta-analysis of five clinical trials, CLE had a pooled sensitivity of 90.4%, specificity of 89.9%, and a NPV of 96.2% for detecting HGD/EAC (28). The high costs associated with CLE and the requirement for administration of an intravenous fluorescent agent have limited widespread adoption. At the present time, the eCLE
system is no longer commercially available, and the only CLE system that currently exists for clinical use is the probe-based variant.Other advanced imaging technologies that are being developed and studied for use in BE include optical coherence tomography (OCT)/volumetric laser endomicroscopy (VLE), autofluorescence imaging (AFI), endocystoscopy, high-resolution microendoscopy (HRME), as well as molecular imaging techniques. The ideal application of advanced imaging in BE would be to combine sensitive, wide-field technologies, with accurate, “optical biopsy” techniques to improve the overall surveillance of BE.
Chemoprevention
Given the poor prognosis of EAC after diagnosis, there has been considerable interest in investigating strategies for disease prevention. One of these areas of investigation is chemoprevention, which has mainly been studied in patients with established BE. The goal of chemoprevention in BE is to avoid the initiation or progression of dysplasia, and/or inhibit the invasion of dysplastic epithelial cells across the basement membrane. The most widely studied classes of drugs for chemoprevention in BE are acid-suppressive agents, non-steroidal anti-inflammatory drugs (NSAIDs), and statins.
Acid suppression
Proton pump inhibitors (PPIs) are the most widely used drugs in the treatment of GERD, and many studies have evaluated their role in Barrett’s chemoprevention, with conflicting results. A large, population-based nested case-control study from the UK concluded that use of acid-suppressive medications on a long-term basis was associated with an increased risk of EAC. However, the authors noted that the association was likely better explained by the fact that the underlying condition for PPI use (i.e., GERD) was itself a risk factor for the development of EAC (34). On the other hand, other prospective and retrospective cohort studies have concluded that PPIs may be protective in patients with BE (35,36). A systematic review and meta-analysis of 7 observational (five cohort and two case-control) studies showed that PPI use was associated with a 71% risk reduction of EAC or HGD in individuals with BE, with a trend towards a dose-response relationship (37). Aggressive acid suppression with a PPI may lead to a partial regression of the specialized IM of BE, but the effect of this on the overall risk of cancer is unknown (38). There are no published studies of chemoprevention following BE ablation.
Aspirin and NSAIDs
The arachidonic acid pathway has been implicated in the carcinogenesis of BE (39). Consequently, there has been much interest in studying the use of cyclo-oxygenase (COX) inhibitors (Aspirin, NSAIDs) as chemopreventive agents in BE. Several observational studies have demonstrated that the use of NSAIDs and/or aspirin in patients with BE is associated with a decreased risk of EAC. A pooled analysis of 6 population-based studies showed that those who used NSAIDs (including aspirin) had a significant risk reduction for the development of EAC (odds-ratio 0.68), with a more pronounced protective effect with increasing duration and frequency of NSAID use (40). However, a randomized trial studying the use of celecoxib in chemoprevention demonstrated that celecoxib did not appear to prevent the transformation of dysplastic BE to EAC (41). A population-based case control study evaluating the use of low-dose aspirin or NSAIDs published in 2015 was also unable to show a reduction in risk of HGD and EAC in patients with BE (42). Given the uncertainty of the currently available data and the potential risks of long-term NSAID use, their use exclusively for the prevention of EAC in patients with BE cannot be recommended at this time (12).
Statins
Statins have also been studied in the chemoprevention of BE. A meta-analysis of 13 observational studies reported a significant reduction (28%) in the risk of esophageal cancer among statin users. In a subgroup of patients with known BE, statins were associated with an even greater (41%) reduction in the risk of EAC (40). A nested case-control study of patients with BE from the national Veterans Affairs (VA) database demonstrated that there was an inverse association between statin use and the development of EAC (adjusted OR 0.65; 95% CI, 0.47–0.91). The protective effect of statins was greater with higher doses and longer duration of use (44). Despite the results from these studies, however, the use of statins for the sole purpose of chemoprevention in BE is not routinely recommended.
Endoscopic therapy of BE
EET has transformed the way we manage dysplastic BE and early (T1a) EAC, and has virtually eliminated the need for surgery in the majority of these patients. EET is recommended in patients with T1a EAC, BE with confirmed HGD, and BE with confirmed LGD. Endoscopic therapy is not recommended in patients with NDBE. The goal of EET is to prevent the progression of dysplastic BE and intramucosal adenocarcinoma to invasive EAC with the goal of reducing morbidity and mortality. There are two main modalities of EET: endoscopic ablation and ER. EET is most successful with a multi-modality approach which combines ER of visible/nodular disease, followed by mucosal ablation of flat BE. Aggressive acid suppression is typically initiated following treatment, and this usually allows for re-epithelization of the esophageal wall with squamous mucosa (neo-squamous epithelium).
Endoscopic ablation
Endoscopic ablation in BE can be performed using thermal energy, photochemical injury, or freezing with the goal of inducing superficial tissue necrosis, followed by healing with neo-squamous epithelium. While endoscopic therapy is currently only recommended in BE with dysplasia, once initiated, the goal of endoscopic ablation is the complete eradication of intestinal metaplasia (CE-IM).
Radiofrequency ablation (RFA)
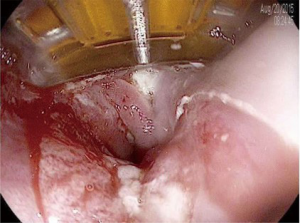
RFA is the most commonly used method of endoscopic ablation in BE. RFA uses a bipolar electrode array and a generator to generate a thermal injury of limited depth (500–1,000 µm) (45). Different sized ablation devices are designed for circumferential (360 degrees) or focal (90 or 60 degrees) RFA. Initial ablation for circumferential BE is typically performed using the 360 degree device, while the focal ablation devices (Figure 3) are typically used for non-circumferential BE and during follow-up “touch-up” sessions, where small residual areas of BE may need to be treated.
In a landmark randomized, sham-controlled trial of RFA for BE with LGD as well as HGD, Shaheen and colleagues reported successful complete eradication of dysplasia (CE-D) in 81–91% of cases in the ablation group vs. 23% of patients in the control group. CE-IM was achieved in 77.4% of patients in the ablation group compared to 2.3% of patients in the sham group. Additionally, there was less neoplastic progression and fewer EACs reported in the RFA group (10). A systematic review and meta-analysis of 18 studies published between 2008 and 2012 examining RFA for BE (NDBE, LGD, and HGD) reported that CE-IM was achieved in 78% and CE-D was achieved in 91% of patients, with recurrence of IM in 13% of patients after eradication (46). A multi-center randomized controlled trial of 136 patients with BE with confirmed LGD compared endoscopic treatment with RFA to endoscopic surveillance and found that ablation reduced the risk of progression to high-grade dysplasia or adenocarcinoma by 25% over a 3-year follow up period as compared to endoscopic surveillance (47). After the first RFA session, patients are typically brought back at 2 month intervals for repeat ablation as needed until CE-IM is achieved. Repeat ablation is performed until all visible Barrett’s tissue has been eradicated (as assessed by inspection under high definition white light endoscopy and electronic chromoendoscopy). Once this is achieved, 4-quadrant biopsies are taken every 1 cm from the entire length of the prior BE segment to confirm CE-IM. Most patients require approximately 3–4 sessions, and this can vary based on the length of the Barrett’s segment. Therefore, RFA has been shown with high-level evidence to significantly increase CE-IM and reduce neoplastic progression and EAC among patients with LGD and HGD.
Initial reports on the durability of RFA for BE were highly encouraging, with one study reporting persistent eradication of dysplasia in 98% of patients and eradication of metaplasia in 91% at the end of 3 years from ablation therapy (48). However, some more recent studies have been less optimistic in terms of the long-term durability of RFA. An analysis from a US multi-center clinical practice consortium reported that 33% of patients had recurrent BE within two years after CE-IM (49), and a study in low risk BE (primarily non-dysplastic) showed recurrence of IM in 50% of patients after CE-IM (50), stressing the need for ongoing surveillance after ablation. It has been demonstrated that there is a strong and significant correlation between endoscopist RFA volume and CE-IM rates (51), so consideration should be given to referring these patients to high-volume centers for the best possible outcomes.
Chest pain after RFA is reported in 25–50% of patients and typically lasts for 3–4 days. The most common serious complication is esophageal strictures in up to 8% of cases; these can usually be successfully managed with endoscopic dilation. Bleeding (<1%) and perforation (<0.01%) are very rare with RFA alone, but can be more common when EMR is performed concurrently with RFA. No procedure-related deaths have been reported (52,53).
A study with data from the U.S. national RFA registry published in 2015 showed that after initiating treatment with RFA in patients with BE, the risk of incident EAC or death from EAC is small (7.8/1,000 person-years and 0.7/1,000 person-years, respectively), with lower EAC incidence rates compared to estimates derived from natural history studies when stratified by baseline histology and degree of dysplasia (54).
Cryotherapy
Cryotherapy involves the application of extremely cold temperatures (−196 °C) using either pressurized liquid nitrogen or carbon dioxide (CO2) in order to achieve tissue injury, disruption of cell membranes, and denaturation of proteins. This is typically done using a spray catheter without direct contact with the mucosa, with the goal being to achieve multiple cycles of rapid freezing and thawing. The use of a decompression tube is usually required to remove excess gas. Typically, 3–4 sessions are needed to completely treat all visible BE.
There are no randomized controlled trials evaluating the efficacy and safety of cryotherapy in BE. The best available evidence is provided from cohort studies. A retrospective cohort study of 96 patients with BE with HGD treated with liquid nitrogen cryotherapy showed that 97% achieved complete eradication of HGD (CE-HGD), 87% had CE-D, and 57% had CE-IM (55). A small study of 32 patients designed to assess the long-term (2 year follow up) safety and efficacy of spray cryotherapy (liquid nitrogen) for BE with HGD demonstrated 100% CE-HGD and 84% CE-IM at 2-year follow-up, with recurrent HGD noted in 16% of patients (56). A prospective cohort study of 80 patients with BE with LGD or HGD from the national cryospray (liquid nitrogen) registry (57) showed CE-D in 91% of patients with LGD and 81% of patients with HGD, while CE-IM was achieved in 61% of patients with LGD and 65% of patients with HGD. A retrospective study of CO2 cryotherapy in 78 patients with neoplastic BE showed similar results, with CE-HGD in 94% of patients and CE-IM in 55% of patients (58). However, CO2 cryotherapy is no longer commercially available. A new balloon-based through-the-scope focal cryoablation device (Figure 4) has been developed to treat BE; clinical trials of the safety and efficacy of this device are ongoing.
There have been no studies directly comparing the efficacy of cryotherapy to that of RFA, and there have been no studies on the cost-effectiveness of cryotherapy compared to other ablative techniques. Cryotherapy has been shown to be a safe technique with rare adverse events, which include stricture formation in 3% of patients (which typically can be managed with endoscopic dilation) and post-procedure chest pain in 2% of patients which is usually self-limited (52).
Photodynamic therapy (PDT)
PDT uses a systemically administered photosensitizing drug, typically prophimer sodium (Ps) 4-aminolevulinic acid (ALA), or m-tetrahydroxyphenyl chlorine, which accumulates in tumor tissue and is then activated by endoscopically delivered laser light using an appropriate wavelength (59). Ps is the most commonly used sensitizer, and is typically given intravenously 2 days prior to the endoscopic procedure to allow time for adequate absorption into the tissue. Once the photosensitizer is activated by the laser light, a photodynamic reaction is initiated and free radicals are produced which lead to cell injury and apoptosis, with deeper tissue penetration compared to RFA or cryotherapy (52).
A large, multi-center, partially blinded randomized controlled trial comparing PDT + PPI to PPI alone in patients with BE with HGD showed rates of CE-HGD of 77% and CE-IM of 52% in the PDT group, but 94% of patients in the PDT group experienced treatment-related adverse events (60). The same group later published long-term follow up of their data and demonstrated that the rate of CE-HGD was maintained at 5 year follow-up (61). A newer retrospective observational study published in 2016 from a single center evaluating PDT vs. RFA + EMR vs. RFA alone in the treatment of BE showed a higher rate of CE-IM at 1 year for the PDT group (71.9%) compared to the RFA + EMR group (47.8%) and the RFA group (22.9%) (62). The main treatment-related adverse events of PDT in the esophagus include skin photosensitivity and esophageal stricture formation. The relatively high stricture rate, up to 36% in some reports (60), and the higher costs of PDT compared to RFA (63) have limited its widespread use. PDT has been largely overtaken by RFA.
ER
ER is typically recommended for visible lesions (either nodular or flat with mucosal irregularity) in BE. ER allows for a definitive histologic analysis (with accurate depth of invasion information) and can often be curative. The two main types of ER are endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
EMR in the esophagus can be performed using different techniques. One method utilizes submucosal fluid injection followed by the use of a cap to facilitate snare resection, and the other uses a banding device to create a “pseudopolyp” which can then be removed with a snare. The latter does not require submucosal fluid injection. Multi-band mucosectomy (MBM) is a variation of the band ligator technique which uses a specialized banding device that contains multiple bands and allows passage of a snare through the banding cap so that multiple resections can be performed in quick succession. MBM has been shown to be quicker and less expensive than the cap technique, with comparable safety and efficacy (64). An analysis of 1,000 patients with intramucosal EAC treated with EMR reported a complete response in 96.3% of patients after a median follow up period of 5 years (65).
ESD was initially developed in Japan to treat early gastric cancer. After submucosal fluid injection, a circumferential incision is performed followed by dissection in the submucosal plane using specialized knives which can be passed through the channel of the endoscope. ESD can be used to achieve en-bloc resection of much larger areas compared to EMR. However, ESD is time-consuming, technically demanding, and associated with increased complications in comparison to EMR. Currently, ESD in the United States is only performed at a small number of specialized centers. A randomized controlled trial from Germany published in 2016 compared EMR (cap technique) to ESD in patients with neoplastic Barrett’s lesions measuring ≤3 cm in size and showed that there was a higher rate of R0 resection (absence of microscopic neoplasia at all the resection margins) with ESD, but no difference in complete remission at 3 months or recurrence rates at 2 year follow-up (66). EMR and ESD are discussed in further detail elsewhere in this issue.
Post-treatment surveillance
Recurrence of BE following CE-IM is not uncommon. A meta-analysis of 41 studies published in 2016 reported that the pooled incidence of BE recurrence (with or without dysplasia/EAC) was 9.5% per patient-year, with rates in individual studies ranging from 0.9% to 28.8%. The same meta-analysis showed that the pooled incidence of dysplastic BE and HGD/EAC was 2.0% and 1.2% per patient-year, respectively. The vast majority of recurrences (95.4%) were successfully treated endoscopically (67). Increasing age and length of BE were found to be predictors of recurrence. A study using data from the U.S. RFA registry reported an overall recurrence rate of 20% after CE-IM, with predictors of recurrence on multivariate analysis being age, length of BE, and non-Caucasian race (68). Therefore, continued endoscopic surveillance after treatment is essential. The intervals and biopsy protocols for post-treatment surveillance are based on expert opinion due to a lack of adequate evidence on which to base recommendations. Guidelines published in 2016 from the American College of Gastroenterology (12) recommend performing surveillance endoscopies every 3 months for the first year following CE-IM, every 6 months in the second year, and then annually in patients treated for HGD or intramucosal adenocarcinoma. In patients treated for LGD, surveillance with upper endoscopy is recommended every 6 months in the first year following CE-IM and annually thereafter. Targeted biopsies of any visible abnormalities should be obtained, in addition to random, 4-quadrant biopsies from the entire length of the prior BE segment. When performing surveillance in the post treatment period, it is important to perform a careful inspection of the tubular esophagus as well as the gastro-esophageal junction (GEJ) in both forward and retroflexed views. Case series have reported occurrence of neoplasia in the cardia or at the GEJ following CE-IM, and surveillance biopsies of the gastric cardia should be routinely performed (12). Some experts also recommend routine ablation of the gastric cardia in patients treated for BE to help reduce recurrence of neoplasia at the cardia, and while this appears to be a reasonable approach, it is not currently supported by evidence.
Post-ablation eosinophilia has been described to occur in 2.7–16% of cases (69,70). While the clinical significance of post-ablation eosinophilia remains uncertain, these studies indicate that none of the patients had clinical signs or symptoms of eosinophilic esophagitis (EoE).
One area of concern in the post-treatment surveillance period is subsquamous intestinal metaplasia (SSIM), more commonly referred to as “buried glands” or “buried Barrett’s.” SSIM is defined as the presence of Barrett’s metaplasia beneath an intact layer of squamous epithelium (Figure 5). SSIM generally cannot be detected by routine endoscopic surveillance, or even by biopsies of the neo-squamous epithelium, as it has been shown that the vast majority of esophageal biopsies are too superficial to detect buried glands (71). While development of EAC from SSIM after RFA has been described at the case report level (72), the early fears that SSIM may be caused by ablation and may increase the risk of developing EAC have not been corroborated. Cohort studies have shown that SSIM is often present prior to ablation in up to 38% of patients (73). It appears that SSIM may actually decrease following ablation of BE. A randomized, sham-controlled trial of RFA in BE noted presence of SSIM in 25% of cases prior to ablation, which decreased to 5% 12 months after ablation (10). A study published in 2015 from the U.S. RFA registry (54) demonstrated that SSIM was associated with an increased risk of EAC, but all subsquamous cancers occurred prior to CE-IM. After CE-IM, SSIM was much less common, suggesting that RFA may eradicate SSIM and prevent subsquamous cancers.
OCT is an imaging modality that allows cross-sectional imaging of superficial layers of tissue to produce images analogous to endoscopic ultrasound (EUS), using light instead of sound. OCT has higher spatial resolution but lower depth of penetration than EUS (74). Its ability to image beneath the surface epithelium has made it an attractive target for study in the evaluation of SSIM. A single-center cross-sectional study found presence of SSIM by OCT in 72% of patients not treated for BE, and in 63% of patients who had achieved CE-IM after RFA, with a significantly lower number of buried glands per patient in the post CE-IM group (75). VLE is a commercially available system based on frequency-domain OCT. A study from the Netherlands published in 2016 evaluating the detection of buried glands after RFA (in patients with HGD or intramucosal EAC) using VLE reported that out of 17 patients, 13 were found to have subsquamous glandular structures by VLE. However, after EMR and histologic evaluation, only 1 out of these 13 subsquamous glandular structures were found to represent SSIM, with the remainder found to be normal histological structures (76). Further data is required regarding the true prevalence, malignant potential, and role of advanced imaging in SSIM before we can make further determinations regarding its true significance.
Conclusions
Endoscopic therapy has become an established standard of care for patients with dysplastic BE. Endoscopic ablation is effective at achieving CE-D and CE-IM in most patients. RFA is the most studied modality and has been shown to be safe and effective in most patients. Due to the remaining and unpredictable risk of recurrence, continued endoscopic surveillance after ablation treatment is required. Most recurrences are amenable to endoscopic treatment with timely detection. Future research should focus on the role of advanced imaging and biomarkers in screening and surveillance both pre and post ablative therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Shiota S, Singh S, Anshasi A, et al. Prevalence of Barrett’s Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2015;13:1907-18. [Crossref] [PubMed]
- Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373:850-61. [Crossref] [PubMed]
- Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am 2015;44:203-31. [Crossref] [PubMed]
- Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2006;4:566-72. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Shakhatreh MH, Duan Z, Kramer J, et al. The incidence of esophageal adenocarcinoma in a national veterans cohort with Barrett’s esophagus. Am J Gastroenterol 2014;109:1862-8; quiz 1861, 1869.
- Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-8. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- di Pietro M, Chan D, Fitzgerald RC, et al. Screening for Barrett’s Esophagus. Gastroenterology 2015;148:912-3. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Saeian K, Staff DM, Vasilopoulos S, et al. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc 2002;56:472-8. [Crossref] [PubMed]
- Shariff MK, Bird-Lieberman EL, O’Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc 2012;75:954-61. [Crossref] [PubMed]
- Peery AF, Hoppo T, Garman KS, et al. Feasibility, Safety, Acceptability and Yield of Office-based, Screening Transnasal Esophagoscopy. Gastrointest Endosc 2012;75:945-953.e2. [Crossref] [PubMed]
- Lao-Sirieix P, Rous B, O’Donovan M, et al. Non-endoscopic immunocytological screening test for Barrett’s oesophagus. Gut 2007;56:1033-4. [Crossref] [PubMed]
- Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372. [Crossref] [PubMed]
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780. [Crossref] [PubMed]
- Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [Crossref] [PubMed]
- Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222-7. [Crossref] [PubMed]
- Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002;122:26-33. [Crossref] [PubMed]
- El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut 2016;65:1252-60. [Crossref] [PubMed]
- Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 2012;76:531-8. [Crossref] [PubMed]
- Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology 1993;105:40-50. [Crossref] [PubMed]
- Anandasabapathy S. Advanced imaging in Barrett’s esophagus: are we ready to relinquish the random? Clin Gastroenterol Hepatol 2013;11:1571-2. [Crossref] [PubMed]
- Mansour NM, Groth SS, Anandasabapathy S. Esophageal Adenocarcinoma: Screening, Surveillance, and Management. Annu Rev Med 2017;68:213-27. [Crossref] [PubMed]
- Sharma P, Savides TJ, Canto MI, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett’s Esophagus. Gastrointest Endosc 2012;76:252-4. [Crossref] [PubMed]
- Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance. Gastrointest Endosc 2016;83:684-698.e7. [Crossref] [PubMed]
- Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560-8. [Crossref] [PubMed]
- Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc 2009;69:1021-8. [Crossref] [PubMed]
- Sharma P, Marcon N, Wani S, et al. Non-biopsy detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a prospective multicenter study. Endoscopy 2006;38:1206-12. [Crossref] [PubMed]
- Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Endoscopy 2010;42:351-9. [Crossref] [PubMed]
- Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: A multicenter international randomized controlled trial (with video). Gastrointest Endosc 2014;79:211-21. [Crossref] [PubMed]
- García Rodríguez LA, Lagergren J, et al. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut 2006;55:1538-44. [Crossref] [PubMed]
- El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877-83. [Crossref] [PubMed]
- Kastelein F, Spaander MC, Steyerberg EW, et al. Proton Pump Inhibitors Reduce the Risk of Neoplastic Progression in Patients With Barrett’s Esophagus. Clin Gastroenterol Hepatol 2013;11:382-8. [Crossref] [PubMed]
- Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229-37. [Crossref] [PubMed]
- Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489-94. [Crossref] [PubMed]
- Baruah A, Buttar NS. Chemoprevention in Barrett’s oesophagus. Best Pract Res Clin Gastroenterol 2015;29:151-65. [Crossref] [PubMed]
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012;142:442-452.e5; quiz e22-3.
- Heath EI, Canto MI, Piantadosi S, et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst 2007;99:545-57. [Crossref] [PubMed]
- Masclee GM, Coloma PM, Spaander MC, et al. NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett’s oesophagus: a population-based case-control study. BMJ Open 2015;5:e006640. [Crossref] [PubMed]
- Singh S, Singh AG, Singh PP, et al. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:620-9. [Crossref] [PubMed]
- Nguyen T, Duan Z, Naik AD, et al. Statin use reduces risk of esophageal adenocarcinoma in US veterans with Barrett’s esophagus: a nested case-control study. Gastroenterology 2015;149:1392-8. [Crossref] [PubMed]
- Belghazi K, Bergman J, Pouw RE. Endoscopic Resection and Radiofrequency Ablation for Early Esophageal Neoplasia. Dig Dis 2016;34:469-75. [Crossref] [PubMed]
- Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for barrett’s esophagus: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FGI, Weusten BLAM, et al. Radiofrequency Ablation vs Endoscopic Surveillance for Patients With Barrett Esophagus and Low-Grade Dysplasia: A Randomized Clinical Trial. JAMA 2014;311:1209. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of Radiofrequency Ablation in Barrett’s Esophagus With Dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
- Gupta M, Iyer PG, Lutzke L, et al. Recurrence of Esophageal Intestinal Metaplasia After Endoscopic Mucosal Resection and Radiofrequency Ablation of Barrett’s Esophagus: Results From a US Multicenter Consortium. Gastroenterology 2013;145:79-86.e1. [Crossref] [PubMed]
- Saligram S, Tofteland N, Wani S, et al. Long-term results of the mucosal ablation of Barrett’s esophagus: efficacy and recurrence. Endosc Int Open 2015;3:E189-194. [Crossref] [PubMed]
- Fudman DI, Lightdale CJ, Poneros JM, et al. Positive correlation between endoscopist radiofrequency ablation volume and response rates in Barrett’s esophagus. Gastrointest Endosc 2014;80:71-7. [Crossref] [PubMed]
- Peter S, Mönkemüller K. Ablative Endoscopic Therapies for Barrett’s-Esophagus-Related Neoplasia. Gastroenterol Clin North Am 2015;44:337-53. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Desai M, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086-1095.e6. [Crossref] [PubMed]
- Wolf WA, Pasricha S, Cotton C, et al. Incidence of Esophageal Adenocarcinoma and Causes of Mortality After Radiofrequency Ablation of Barrett’s Esophagus. Gastroenterology 2015;149:1752-1761.e1. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc 2013;78:260-5. [Crossref] [PubMed]
- Ghorbani S, Tsai FC, Greenwald BD, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s dysplasia: results of the National Cryospray Registry. Dis Esophagus 2016;29:241-7. [Crossref] [PubMed]
- Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:591. [Crossref] [PubMed]
- Shishkova N, Kuznetsova O, Berezov T. Photodynamic therapy in gastroenterology. J Gastrointest Cancer 2013;44:251-9. [Crossref] [PubMed]
- Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [Crossref] [PubMed]
- Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc 2007;66:460-8. [Crossref] [PubMed]
- David WJ, Qumseya BJ, Qumsiyeh Y, et al. Comparison of endoscopic treatment modalities for Barrett’s neoplasia. Gastrointest Endosc 2015;82:793-803.e3. [Crossref] [PubMed]
- Ertan A, Zaheer I, Correa AM, et al. Photodynamic therapy vs radiofrequency ablation for Barrett’s dysplasia: efficacy, safety and cost-comparison. World J Gastroenterol 2013;19:7106-13. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660.e1. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090-1106.e3. [Crossref] [PubMed]
- Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840-1847.e1. [Crossref] [PubMed]
- Halsey KD, Arora M, Bulsiewicz WJ, et al. Eosinophilic infiltration of the esophagus following endoscopic ablation of Barrett’s neoplasia. Dis Esophagus 2013;26:113-6. [Crossref] [PubMed]
- Villa N, El-Serag HB, Younes M, et al. Esophageal eosinophilia after radiofrequency ablation for Barrett’s esophagus. Dis Esophagus 2013;26:674-7. [PubMed]
- Gupta N, Mathur SC, Dumot JA, et al. Adequacy of esophageal squamous mucosa specimens obtained during endoscopy: are standard biopsies sufficient for postablation surveillance in Barrett’s esophagus? Gastrointest Endosc 2012;75:11-8. [Crossref] [PubMed]
- Chabrun E, Marty M, Zerbib F. Development of esophageal adenocarcinoma on buried glands following radiofrequency ablation for Barrett’s esophagus. Endoscopy 2012;44 Suppl 2 UCTN:E392.
- Sharma P, Morales TG, Bhattacharyya A, et al. Squamous Islands in Barrett’s Esophagus: What Lies Underneath? Am J Gastroenterol 1998;93:332-5. [Crossref] [PubMed]
- Muthusamy VR, Kim S, Wallace MB. Advanced Imaging in Barrett’s Esophagus. Gastroenterol Clin North Am 2015;44:439-58. [Crossref] [PubMed]
- Zhou C, Tsai TH, Lee HC, et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc 2012;76:32-40. [Crossref] [PubMed]
- Swager AF, Boerwinkel DF, de Bruin DM, et al. Detection of buried Barrett’s glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointest Endosc 2016;83:80-8. [Crossref] [PubMed]