Non-functional tricuspid valve disease
Review of non-functional tricuspid regurgitationOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
More than 75% of severe tricuspid regurgitation is classified as functional or related to pulmonary hypertension or right ventricular dysfunction or dilatation, rather than independent abnormalities of the tricuspid valve components: leaflets, annulus, chordal attachments, and papillary muscles. An understanding of non-functional tricuspid regurgitation mandates an appreciation of functional tricuspid regurgitation because the former may ultimately be worsened by some of the same forces that propel the latter (1,2).
The anatomy of the tricuspid valve lends itself to functional impairment. Unlike the dense, separate, fibrous structure, with two fibrous trigones, that surrounds the mitral valve, the tricuspid annulus, with only a right fibrous trigone, blends into the right atrial fibro fatty structures and is more susceptible to dilate or not reduce appropriately in size during systole, with either right atrial or right ventricular dilatation. Only two papillary muscles, albeit with an additional posterior commissural chord, supply three leaflets. The chordal attachments to the anterior leaflet arise largely from the anterolateral papillary muscle, not both papillary muscles, and there is a reliance on attachments to the moderator band and free wall. The smallest leaflet, the septal leaflet, is relatively fixed. Right ventricular expansion can readily tether or restrict the anterior leaflet. The posterior leaflet can become restricted via an interesting mechanism: the posteroseptal portion of the valve is more apically displaced than the anterior portion, and right ventricular dilatation, which allows pulling on the anterior portion of the valve in an apical direction, makes the valve more planar, thus positioning the posterior leaflets in a tethered position (3-6).
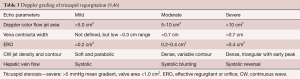
This review will discuss the multiple etiologies of tricuspid regurgitation that are not initially related to either pulmonary hypertension or right ventricular dilation (Table 1). After a description of the entities, there will be a discussion of the progression and prognosis of severe non-functional tricuspid regurgitation and how symptoms, examination, tricuspid regurgitation grading, and associated structural changes reflect the blending of isolated tricuspid regurgitation into functional tricuspid regurgitation under chronic conditions. Last, there will be a discussion of recommended treatment strategies.

Full table
Entities with substantial leaflet pathologyOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Rheumatic disease
The tricuspid valve ranks third behind the mitral and aortic valves in terms of likelihood of developing severe rheumatic disease pathology. The leaflets become thickened, fibrotic, and retracted; there is commissural fusion; and the rheumatic inflammation can also involve the chordae, contributing further to restriction of the leaflets. There is resultant tricuspid stenosis and regurgitation with regurgitation predominant (10).
Rheumatic disease dominates early series and plays an important role even in more recent series that discuss the pathology found at the time of isolated tricuspid valve surgery (2,11,12), but nearly all of the patients characterized had previously undergone mitral valve surgery. Isolated tricuspid surgery in rheumatic patients is purported to carry a high risk (12), but the risk is related to a combination of factors including age, reoperation, right ventricular impairment, and unresolved pulmonary hypertension.
Myxomatous or degenerative disease
Myxomatous disease or degenerative disease, with either a Barlow’s or fibroelastic insufficiency appearance and that encompasses connective tissue diseases like Marfans or Ehlers-Danlos, plays a prominent role in some series of severe, isolated tricuspid surgery (13). The myxomatous process not only involves leaflets, but chordae, and the annulus. Myxomatous related annular dilatation contributes not only to the degree of tricuspid regurgitation, but to progressive worsening of tricuspid regurgitation. As with rheumatic disease, the vast majority of isolated myxomatous tricuspid valve surgery occurs in patients who previously underwent left-sided surgery. The prognosis is related to right ventricular function as well as residual pulmonary hypertension. Rheumatic and myxomatous left-sided surgery without requiring immediate attention to the tricuspid valve may be associated with the development of 3+ or greater tricuspid regurgitation in 16% of patients over an 8-year period, and the distinction between non-functional and functional contributions to tricuspid valve regurgitation severity may blur (14).
Endocarditis
Bacterial endocarditis can destroy or perforate leaflets and rupture chordae, often atop already abnormal valves that are floppy or rheumatic. Large vegetations can be associated with relative stenosis (7), although regurgitation generally predominates. Prognosis depends upon degree of perivalvular damage, abscess formation, valve destruction, right ventricular failure, emboli, organism, removal of a device or catheter if present, and in the case of drug abusers, change in behavior. Rarely, the tricuspid valve, and not just the aortic valve, can be made severely regurgitant by a typically culture-negative organism, Tropheryma whipplei, which can form a leaflet plaque and thus restriction of leaflets. This restriction is associated with either stenosis or regurgitation that may resolve with appropriate antibiotic treatment (7,15).
Sarcoid
Sarcoid is a rare cause of isolated tricuspid regurgitation. Granulomas has been found in the annular region and papillary muscles, as well as on excised valves (12). Hallmarks of cardiac sarcoid include conduction system disease and arrhythmias, as well as widespread left ventricular and more focal right ventricular scaring. Prognosis depends on control of the disease. While the inflammatory process can be arrested and conduction disease can sometimes improve with treatment, valvular destruction is less likely to improve, just as left ventricular damage does not resolve (16,17).
Lupus
Approximately half of patients who have systemic lupus erythematosus have cardiac involvement with immunopathologic changes that can involve myocardium, pericardium, coronary arteries, and valves. Of the patients who have cardiac involvement, valves are involved in as many as 40%, with the tricuspid valve much less likely to be involved than the left-sided valves (18,19). The valvular pathology in lupus includes immune deposition, mononuclear infiltration, fibrous plaque, calcinosis and particularly with the IgG antiphospholipid antibody syndrome, thrombi or Libman-Sacks verrucous endocarditis. The bulky thrombi can cause stenoses, but rarely severe valve destruction. The fibrotic reaction can thicken the valve as well as chordae and cause leaflet fusion and fibrosis with important tricuspid regurgitation or even tricuspid stenosis. The prognosis depends upon control of the underlying disease, which can sometimes mitigate the valvular disease, in addition to the presence of left-sided disease and pulmonary hypertension. Surgical intervention is sometimes required. Antiphospholipid antibody syndrome, even in the absence of SLE, can cause what is likely non-damaging verrucous tricuspid regurgitation (20).
Implanted endocardial electrical devices
Permanent pacing and ICD leads are associated approximately one quarter of the time with an increase in tricuspid regurgitation by one grade (21) and more rarely, with tricuspid stenosis (22). The pathology includes fibrosis and fusion of the wire to leaflets and the sub valvular structures, as well as perforation and even dynamic looping of a leaflet that has been reported to cause resting stenosis that can improve with inspiration and exercise (23). The presence of a permanent pacing wire at the time of tricuspid valve repair more than doubles the risk for moderate and severe tricuspid regurgitation by five years post-operatively (24). The worsening tricuspid regurgitation can also have a functional component related to hemodynamic and structural alterations from right ventricular pacing.
Carcinoid
Carcinoid tumors that produce the carcinoid syndrome with flushing, diarrhea, and sometimes wheezing, more than half of the time, with a long latency period (49±70 months) can cause thickened, retracted, short, hypomobile, rigid tricuspid leaflets and similar pulmonic valve pathology. The tricuspid valve can be regurgitant, stenotic, or mixed (25). The valve damage is related to circulating levels of 5HIAA, which acts on the 5 hydroxytryptamine receptor subtype 5-HT2B, a mediator of mitogenesis and fibroblast proliferation (26). High levels of 5HIAA are more likely to be present when there are hepatic metastases. The carcinoid lesions are not known to regress, even with control of the disease, but prognosis is still related to the ability to treat the underlying disease and timely tricuspid valve surgery. Careful attention to the pulmonic valve at the time of tricuspid surgery is associated with an improvement in functional class, right ventricular size, and prognosis (27).
Ergot alkaloids and fenfluramine
The ergot alkaloid methysergide, used in the past for migraine, and diet pills containing fenfluramine, as well as the ergot derived dopamine receptor agonists, cabergoline utilized to treat pituitary tumors and pergolide, utilized for Parkinson’s disease, have been associated with carcinoid like or 5-HT2B activated valvular pathology. The effect of these agents on the tricuspid valve is generally related to dose and duration of continuous exposure. With methysergide, thickening can involve chordae and the papillary muscle and may be severe (28). With fenfluramine, the incidence of tricuspid valve involvement is less than left-sided involvement, and the incidence of any type of valvular involvement, although reported in up to 30–60% of patients, is in the range of 2–12% in studies done with a control group. Even seemingly severe regurgitant disease, usually associated with an exposure to fenfluramine for approximately one year, frequently regresses when the offending agent is stopped (29-31). The relative risk for development of severe tricuspid regurgitation for the ergot dopamine receptor agonists is between 5 and 18, depending on the control populations, and is less than the risk for left sided valves (26,32).
Radiation
In a series of 415 Hodgkin Lymphoma mantle irradiation survivors, 6.2% developed moderate to severe valvular disease, many requiring surgery, with a mean time from radiation of 22 years in an observed:expected ratio (O:E) of 8.42 (less frequent, longer latency, and lower O:E than for coronary artery disease—10.4%, 9 years, 1.63, respectively). While many patients with aortic and mitral valve disease required surgery, the three patients with significant tricuspid regurgitation did not undergo surgery (33). The etiology of the tricuspid regurgitation could have been partly functional, related to radiation-induced alterations in right ventricular function; left ventricular systolic or diastolic function; or pulmonary hypertension. The mean radiation dose was 37 Gy. In breast cancer survivors, the O:E for valvular disease was elevated, 3.17 (1.9–5.29, P<0.001), but only in patients who received either side internal mammary chain radiation. The incidence of tricuspid valve disease, which could have a functional contribution, is not reported (34,35).
Blunt traumaOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Blunt trauma, usually from a motor vehicle accident, and sometimes from an airbag injury, can cause acute compression, and thus a rapid elevation, in right ventricular pressure as well as a deceleration injury. Autopsy findings from victims of blunt cardiac injury suggest that while the right ventricle may be injured 40% of the time, tricuspid valve injury is much rarer and is seen in only 3% of cases (36). There can be anterior leaflet tears that might be associated with a period of stability, anterior papillary muscle damage with immediate or delayed heart-failure symptoms, or chordal rupture that can be associated with a long period of stability (37). Suspicion, close follow up and early surgery in the setting of acute right ventricular failure, can lead to good outcomes.
Congenital defects
Multiple congenital defects, listed in Table 1, can cause non-functional tricuspid regurgitation. Ebstein’s anomaly accounted for 39 of 70 congenital patients undergoing isolated tricuspid valve surgery (11). Other congenital lesions, including tricuspid valve hypoplasia and dysplasia, cleft, and double orifice, often with multiple accompanying congenital defects (8) are outside the scope of this review.
Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertensionOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
In addition to sarcoid and myxomatous disease, including connective tissue diseases like Ehlers-Danlos and Marfan’s, both already discussed, atrial fibrillation can also cause annular dilatation and tricuspid regurgitation.
Atrial fibrillation
During atrial systole, the tricuspid annular dimension is known to decrease by nearly 20%, so atrial fibrillation alone can set into motion a cycle of regurgitation, atrial and annular dilatation, and worsened regurgitation. In two series of severe tricuspid regurgitation, over 85% of “idiopathic” annular dilatation patients had atrial fibrillation (1,38).
Entities that affect chordae and papillary musclesOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
The Table 1 items in this category have all been discussed except Loeffler’s endocardial fibroelastosis (Loeffler’s) and endomyocardial biopsy.
Endocardial fibroelastosis
Endocardial fibroelastosis is generally associated with an eosinophil count of 1,500/mm3 for more than six months and can be “tropical” with eosinophilic endomyocardial fibrosis or non-tropical with eosinophilic myocarditis (39). Both sides of the heart can be involved. The right ventricular endocardium, papillary muscles, chordae and tricuspid leaflets can be encased in fibrosis, with extensive thrombus attachment and consequent valve restriction with either significant regurgitation or stenosis (40). Treatment of the underlying disease that causes the eosinophilia can lead to important improvement in valve function if treatment is initiated before permanent damage from the toxic products of eosinophil degranulation.
Endomyocardial biopsy
In 364 heart transplant patients undergoing multiple endomyocardial biopsies over a span of 10 years, 54 developed flail tricuspid leaflets related to severing of chordal attachments. Over a two year period, right ventricular sphericity, end diastolic area and annular size increased, demonstrating the continuum from non-functional to a mixture of non-functional and functional tricuspid regurgitation (41).
Intra annular or periannular obstruction or impingementOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Renal and ovarian tumors can grow into the tricuspid orifice creating stenosis or regurgitation. Atrial myxomas or other cardiac tumors, or even a large sinus of valsalva aneurysm, could impinge upon the valve and cause severe annular distortion and tricuspid regurgitation (7). Treatment is directed at the tumor or aneurysm and generally includes excision.
PrognosisOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Medical management of moderate to severe tricuspid regurgitation, after adjustment for right ventricular size, left ventricular ejection fraction, and pulmonary pressure is associated with a 4–5-year 30–50% increased mortality compared to patients with no tricuspid regurgitation (42-44).
Several lines of evidence suggest that severe tricuspid regurgitation, even in the absence of pulmonary hypertension or initial RV dysfunction, leads to RV dilatation, insidious loss of RV reserve despite an apparent preserved RV ejection fraction, the potential for unforeseen unmasking of significant RV dysfunction at the time of tricuspid valve surgery, and poor survival. The creation of severe tricuspid regurgitation at the time of right ventricular biopsy is associated with a >20% increase in right ventricular transverse diameter over a two year period, even in the absence of pulmonary hypertension (41), though the right ventricular ejection fraction remains excellent. A quantitative preoperative RV assessment in patients with severe tricuspid regurgitation showed that even when there appeared to be a normal right ventricular ejection fraction, careful calculation of percent area change of the right ventricle was already abnormal; 26.6%±6.74%, with normal being above 40% (13). When patients who had a normal appearing right ventricle, but a tricuspid annular dimension >40 mm (normal <34 mm), underwent mitral valve surgery and tricuspid valve repair, despite some degree of presumed RV unloading, with a fall in right ventricular systolic pressure from a mean of 38 mmHg to a mean of 29 mmHg, only 30% of the patients had a normal right ventricular ejection fraction postoperatively. Right ventricular function required 3.5–5 years to recover (45). In patients with generally preserved RV ejection fraction and only a load independent sign of right ventricular loss of reserve, a right ventricular end systolic area greater than 20 cm2, or another relatively subtle sign of RV impairment, hypersplenism, with a hemoglobin less than 11.3 g/dL, isolated tricuspid valve surgery was associated with a two-year survival in the 44–57% range (2).
As would be expected, if there is overt right heart failure, the post-operative results can be poor. In patients who underwent isolated tricuspid valve surgery for severe tricuspid regurgitation, among whom the incidence of heart failure was >70%, with a mean right atrial pressure of 17 mmHg, mean pulmonary artery pressure 27 mmHg, the operative mortality was 20% and after 3 years, the survival rate was only 40% (12).
The prognosis for patients with severe, non-functional tricuspid regurgitation hinges on early detection and close follow up, which involves quantitative grading of tricuspid regurgitation and RV function, and intervention while right ventricular function (percent area change 35% or greater, and end systolic area less than 20 cm2) is good. Two year survival rates above 90% have been shown in patients who showed no sign of RV impairment, excellent right ventricular end systolic area, <20 cm2 and no signs of hypersplenism (hemoglobin above 11.3 g/dL) (2).
Once there is right ventricular failure or even a subtle loss of reserve, even after intervention, right ventricular function might plummet, with a slow recovery, or might not even recover enough to afford reasonable longevity.
SymptomsOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
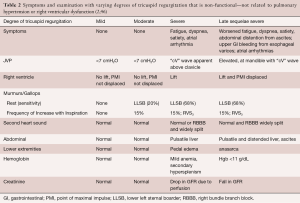
The symptoms (Table 2) associated with severe tricuspid regurgitation, shortness of breath and fatigue, related to poor forward output, might initially be present when only right ventricular exercise reserve is impaired. However, more often, the symptoms become evident much later when there is much worse right ventricular function, replete with right ventricular failure, and thus resting fatigue as well as early satiety, and lower extremity swelling. The symptoms could be confounded by the diseases causing the non-functional tricuspid regurgitation, such as SLE, carcinoid, or sarcoid. Similarly, the exam (Table 2) may be overt and revealing only when the presentation is late, with nearly manifest or fully manifest right ventricular failure, with cardiac cirrhosis, ascites, anasarca, and esophageal varices There can be a full volume, high “cV” wave, diminished carotid volume, right ventricular lift, lower left sternal border systolic murmur, right ventricular S3 with inspiration, pulsatile or displaced downward liver, and lower extremity swelling. Even with severe tricuspid regurgitation, the systolic murmur may be heard only two-thirds of the time, with change in inspiration only 15% of the time (43).
Full table
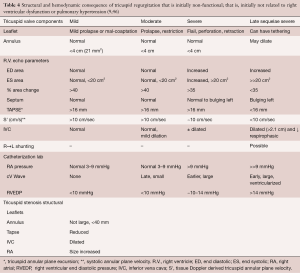
Echocardiography with quantification of tricuspid regurgitation is paramount to following tricuspid regurgitation closely (Table 3). ERO >0.4 cm2 and vena contracta >0.7 cm, which correlate well with each other (R 0.81; P<0.01) (38), combined with systolic hepatic venous flow reversal and the characteristic triangular shaped continuous wave Doppler, define severe tricuspid regurgitation and thus highlight a group of patients at high risk for progressive right ventricular decompensation and perhaps irreversible or exceedingly slowly reversible right ventricular function. Non-functional tricuspid regurgitation without intervention is likely to cause progressive annular dilatation and worsened tricuspid regurgitation with progressive right ventricular enlargement and dysfunction, as outlined in Table 4, that details right ventricular volumes, tricuspid annular plane systolic excursion, and other parameters reflective of right ventricular performance.
Full table
InterventionsOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
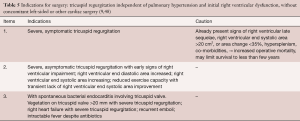
Not every type of non-functional tricuspid regurgitation requires operative intervention. Treatment of SLE and antiphospholipid antibody syndrome may reduce the “coating” over the valves and chordae and reduce stenosis and regurgitation. Cessation of fenfluramine or methysergide has been associated with valve normalization (28,29,31). Severe tricuspid regurgitation from a Whipple’s plaque that seemed to coat the tricuspid valve leaflets without destroying them, reportedly resolved with antibiotics (15). There are reports of heart failure symptom improvement with attention to carcinoid, but the valvular pathology and severity of the underlying pathologic change in fact may actually worsen despite treatment of the disease (47). Endomyocardial fibroelastosis, prior to severe eosinophilic damage, may be associated with reversible severe tricuspid regurgitation. The other entities in Table 1 are not likely to be associated with reversal of severe tricuspid regurgitation. The standard dictum, to await symptoms, try diuretics and then try surgery, may be too conservative, and may worsen right ventricular function, increase operative mortality risk, and worsen long-term prognosis. Reasonable proposals for intervention generally and in the setting of tricuspid endocarditis are listed in Table 5 (9,48).
Full table
Valve repairs with a rigid ring may be preferable to valve replacement. If replacement is needed, common thinking favors bioprosthesis, without definite evidence for superiority, based on an analysis of 13 series (49) and with a high percentage of patients with a bioprosthesis remaining on long-term anticoagulation. There are reports of percutaneous solutions, outside the scope of this review, but some involve creative use of a large stent and then a percutaneous valve (in absence of a previously placed prosthetic ring) (50) and a novel bicuspid technique (51).
ConclusionsOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Multiple entities affect the tricuspid leaflets, annulus, chordae and papillary muscles and can cause severe tricuspid regurgitation or stenosis in the initial absence of either pulmonary hypertension or right ventricular dysfunction. Over time, these entities can cause progressive right atrial, right ventricular, and annular dilatation, sometimes with atrial fibrillation. They may also be associated with signs of poor forward output and right ventricular heart failure, with structural manifestations similar to what is seen in the much more common entity of functional tricuspid regurgitation. Since pulmonary hypertension reduction and consequent right ventricular unloading do not generally occur after intervention on isolated, non-functional tricuspid regurgitation, non-functional tricuspid regurgitation has a poor surgical outcome when the surgery is done when right ventricular function is already impaired. Careful attention to quantitative echo grading and the slightest symptoms, examination, right ventricular structural alterations, and timely surgery, is mandatory for the treatment of patients with most types of non-functional tricuspid regurgitation.
AcknowledgementsOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
None.
FootnoteOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The author has no conflicts of interest to declare.
ReferencesOther Section
- Review of non-functional tricuspid regurgitation
- Entities with substantial leaflet pathology
- Blunt trauma
- Entities with substantial annular pathology, not attributable to right ventricular alteration or pulmonary hypertension
- Entities that affect chordae and papillary muscles
- Intra annular or periannular obstruction or impingement
- Prognosis
- Symptoms
- Interventions
- Conclusions
- Acknowledgements
- Footnote
- References
- Mutlak D, Lessick J, Reisner SA, et al. Echocardiography-based spectrum of severe tricuspid regurgitation: the frequency of apparently idiopathic tricuspid regurgitation. J Am Soc Echocardiogr 2007;20:405-8. [Crossref] [PubMed]
- Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672-8. [Crossref] [PubMed]
- Rogers JH, Bolling SF. The Tricuspid Valve: Current Perspective and Evolving Management of Tricuspid Regurgitation. Circulation 2009;119:2718-25. [Crossref] [PubMed]
- Anderson, RH, Becker, AE. Slide Atlas of Cardiac Anatomy 10: The Development of the Heart. Slide Atlas of Cardiac Anatomy 10: The Development of the Heart. s.l.: Gower Medical Publishing Ltd., 1980.
- Fukuda S, Saracino G, Matsumura Y, et al. Three-Dimensional Geometry of the Tricuspid Annulus in Healthy Subjects and in Patients With Functional Tricuspid Regurgitation. Circulation 2006;114:I492-8. [Crossref] [PubMed]
- Tei C, Pilgrim JP, Shah PM, et al. The tricuspid valve annulus: study of size and motion in normal subjects and in patients with tricuspid regurgitation. Circulation 1982;66:665-71. [Crossref] [PubMed]
- Waller BF, Howard J, Fess S. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation--Part I. Clin Cardiol 1995;18:97-102. [Crossref] [PubMed]
- Bruce CJ, Connolly HM. Right-sided valve disease deserves a little more respect. Circulation 2009;119:2726-34. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- Cheitlin MD, MacGregor JS. Aquired Tricuspid and Pulmonary Valve Disease. In: Topol EJ. Editor. Textbook of Cardiovascular Medicine. Philadelphia: Lippincott-Raven Publishers, 1998.
- Hauck AJ, Freeman DP, Ackermann DM, et al. Surgical pathology of the tricuspid valve: a study of 363 cases spanning 25 years. Mayo Clin Proc 1988;63:851-63. [Crossref] [PubMed]
- Mangoni AA, DiSalvo TG, Vlahakes GJ, et al. Outcome following isolated tricuspid valve replacement. Eur J Cardiothorac Surg 2001;19:68-73. [Crossref] [PubMed]
- Mukherjee D, Nader S, Olano A, et al. P. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation. J Am Soc Echocardiogr 2000;13:650-4. [Crossref] [PubMed]
- Matsuyama K, Matsumoto M, Sugita T, et al. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826-8. [Crossref] [PubMed]
- Gabus V, Grenak-Degoumois Z, Jeanneret S, et al. Tropheryma whipplei tricuspid endocarditis: a case report and review of the literature. J Med Case Rep 2010;4:245. [Crossref] [PubMed]
- Goyal SB, Aragam JR. Cardiac sarcoidosis with primary involvement of the tricuspid valve. Cardiol Rev 2006;14:e12-3. [Crossref] [PubMed]
- Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol 2015;8:87-93. [Crossref] [PubMed]
- Ames DE, Asherson RA, Coltart JD, et al. Systemic lupus erythematosus complicated by tricuspid stenosis and regurgitation: successful treatment by valve transplantation. Ann Rheum Dis 1992;51:120-2. [Crossref] [PubMed]
- Gabrielli F, Alcini E, Di Prima MA, et al. Cardiac valve involvement in systemic lupus erythematosus and primary antiphospholipid syndrome: lack of correlation with antiphospholipid antibodies. Int J Cardiol 1995;51:117-26. [Crossref] [PubMed]
- Reshetniak TM, Kotel'nikova GP, Fomicheva OA, et al. Cardiological aspects of the antiphospholipid syndrome. Part I. Valvular lesions in the primary and secondary antiphospholipid syndrome and systemic lupus erythematosus. Kardiologiia 2002;42:38-43. [PubMed]
- Kim JB, Spevack DM, Tunick PA, et al. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr 2008;21:284-7. [Crossref] [PubMed]
- Al-Hijji M, Yoon Park J, El Sabbagh A, et al. The Forgotten Valve: Isolated Severe Tricuspid Valve Stenosis. Circulation 2015;132:e123-5. [Crossref] [PubMed]
- Skoric B, Baricevic Z, Brida M, et al. Dynamic tricuspid valve stenosis induced with a pacemaker lead: a case report. J Heart Valve Dis 2014;23:142-4. [PubMed]
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674-85. [Crossref] [PubMed]
- Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 1993;87:1188-96. [Crossref] [PubMed]
- Zanettini R, Antonini A, Gatto G, et al. Valvular Heart Disease and the Use of Dopamine Agonists for Parkinson's Disease. N Engl J Med 2007;356:39-46. [Crossref] [PubMed]
- Connolly HM, Schaff HV, Mullany CJ, et al. Carcinoid heart disease: impact of pulmonary valve replacement in right ventricular function and remodeling. Circulation 2002;106:I51-I56. [PubMed]
- Mason JW, Billingham ME, Friedman JP. Methysergide-induced heart disease: a case of multivalvular and myocardial fibrosis. Circulation 1977;56:889-90. [Crossref] [PubMed]
- Mast ST, Jollis JG, Ryan T, et al. The progression of fenfluramine-associated valvular heart disease assessed by echocardiography. Ann Intern Med 2001;134:261-6. [Crossref] [PubMed]
- Weissman NJ. Appetite suppressants and valvular heart disease. Am J Med Sci 2001;321:285-91. [Crossref] [PubMed]
- Seghatol FF, Rigolin VH. Appetite Suppressants and Valvular Heart Disease . Curr Opin Cardiol 2002;17:486-92. [Crossref] [PubMed]
- Izgi C, Feray H, Cevik C, et al. Severe tricuspid regurgitation in a patient receiving low-dose cabergoline for the treatment of acromegaly. J Heart Valve Dis 2010;19:797-800. [PubMed]
- Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831-7. [Crossref] [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [Crossref] [PubMed]
- Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006;24:4100-6. [Crossref] [PubMed]
- de Biasi AR, Seastedt KP, Eachempati SR, et al. Common Cause of Mortality in Trauma but Manageable Nonetheless. Circulation 2015;132:537-45. [Crossref] [PubMed]
- Dounis G, Matsakas E, Poularas J, et al. Traumatic tricuspid insufficiency: a case report with a review of the literature. Eur J Emerg Med 2002;9:258-61. [Crossref] [PubMed]
- Yang WI, Shim CY, Kang MK, et al. Vena contracta width as a predictor of adverse outcomes in patients with severe isolated tricuspid regurgitation. J Am Soc Echocardiogr 2011;24:1013-9. [Crossref] [PubMed]
- Benezet-Mazuecos J, de la Fuente A, Marcos-Alberca P, et al. Loeffler endocarditis: what have we learned? Am J Hematol 2007;82:861-2. [Crossref] [PubMed]
- Kline, A, Scalia, G. Diseases of the Pericardium, Restrictive Cardiomyopathy and Diastolic Dysfunction. In: Topol EJ. Editor. Textbook of Cardiovascular Medicine. Philadelphia, NY: Lippincott-Raven, 1998.
- Reynertson SI, Kundur R, Mullen GM, et al. Asymmetry of right ventricular enlargement in response to tricuspid regurgitation. Circulation 1999;100:465-7. [Crossref] [PubMed]
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185-94. [Crossref] [PubMed]
- Messika-Zeitoun D, Thomson H, Bellamy M, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg 2004;128:296-302. [Crossref] [PubMed]
- Chikwe J, Itagaki S, Anyanwu A, et al. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J Am Coll Cardiol 2015;65:1931-8. [Crossref] [PubMed]
- Geske JB, Scantlebury DC, Thomas JD, et al. Hemodynamic evaluation of severe tricuspid regurgitation. J Am Coll Cardiol 2013;62:e441. [Crossref] [PubMed]
- Goldman T, Adamson K, Yang E. Resolution of right-sided heart failure symptoms after resection of a primary ovarian carcinoid tumor. Tex Heart Inst J 2014;41:533-6. [Crossref] [PubMed]
- Poterucha T, Vedula R, Kapur S, et al. No Right Answer. Circulation 2015;132:2259-64. [Crossref] [PubMed]
- Kunadian B, Vijayalakshmi K, Balasubramanian S, et al. Should the tricuspid valve be replaced with a mechanical or biological valve? Interact Cardiovasc Thorac Surg 2007;6:551-7. [Crossref] [PubMed]
- Kefer J, Sluysmans T, Vanoverschelde JL. Transcatheter Sapien valve implantation in a native tricuspid valve after failed surgical repair. Catheter Cardiovasc Interv 2014;83:841-5. [Crossref] [PubMed]
- Schofer J, Bijuklic K, Tiburtius C, et al. First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J Am Coll Cardiol 2015;65:1190-5. [Crossref] [PubMed]