Hypertrophic obstructive cardiomyopathy—the Leipzig experience
Introduction
Functional obstruction of the left ventricle (LV) was first mentioned by RC Brock in 1957 (1). Hypertrophic cardiomyopathy (HCM) is a descriptive term for cardiomyopathies with myocardial thickness ≥15 mm in one or more myocardial LV segments (2). Around 60% of HCM are autosomal dominant and are caused by mutations of the cardiac sarcomere protein. Other causes of hypertrophy include metabolic storage disease, neuromuscular disorders and non-genetic diseases such as transthyretin amyloidosis. The prevalence is estimated at 0.02% of adults in the developed world (3). HCM affects both genders with a small male preponderance (4). Left ventricular outflow tract obstruction (LVOTO) is defined as ventricular gradient of greater than 30 mmHg. It occurs in 25% of hypertrophic cardiomyopathies at rest (5) and can affect an additional 30% of patients during exercise (dynamic LVOTO) (2). Obstruction is caused either by hypertrophied myocardium or systolic anterior movement (SAM) of the mitral valve (MV) leaflet. Symptoms of outflow obstruction usually occur at gradients greater than 50 mmHg, which include dyspnea, chest pain and syncope (6). HCM may cause arrhythmias, heart failure and sudden cardiac death. The annual incidence of sudden cardiac death in patients with HCM is 1–2% (2), and is the most common cause of sudden death in young competitive athletes (7,8). If the LVOTO exceeds a gradient of 50 mmHg at rest or under exercise, stress echocardiography in symptomatic patients and invasive therapy of LVOTO should be considered. Over the last few years, transcoronary ablation of septal hypertrophy (TASH) has been established as an alternative to septal myectomy (SM). Although surgical and septal ablation have not been compared in a randomized trial, the general consensus is that surgical treatment should be offered to patients with significant septal thickness (>17 mm) and additional valvular pathologies or coronary artery disease that could be addressed at the same time (6,9). In 1961, Morrow and Brockenbrough described the surgical treatment of hypertrophic obstructive cardiomyopathy (HOCM) (10). In this article, we report our single-center experience in the surgical management of HOCM.
Methods
We searched the surgical database for patients who underwent SM at our institution between 1997 and 2016. Patients, who underwent myocardial resections for other reasons than HCM were excluded from the analysis. Patient demographics, investigations and treatments were obtained from our database. HOCM was diagnosed by transthoracic echocardiography. Pre- and postoperative transthoracic echocardiographic data were acquired and analyzed by an experienced echocardiographer using current guidelines to assess cardiac function (11). The degree of myectomy to relieve LVOT is varied upon the extent and the nature of the obstruction, ranging from local subaortic to extensive myectomy of nearly the entire LV cavity (10,12).
All patients underwent myectomy under cardiopulmonary bypass (CPB). Intraoperative transthoracic echocardiography was used to monitor the extent and level of LVOT, septal thickness and possible abnormalities of the MV after weaning from CPB. Follow up data was collected through telephone interviews at various postoperative time points to establish whether the patients were alive or dead. Patients with absent data were excluded from our analysis.
Statistical analysis
Standard definitions were used for patient variables and outcomes. Categorical variables were expressed as percentages, and continuous variables as mean ± SD (range). All statistical analyses were performed using IBM SPSS v. 24.0 software (IBM Corp., New York, USA). Comparisons of the preoperative and follow-up results were performed using a two-paired t-test and the Wilcoxon signed rank test respectively. Long-term survival of groups was evaluated and compared using the Kaplan-Meier Survival plot and the log-rank test. A two-sided P value <0.05 was considered to be statistically significant.
Results
From 1997 to 2016, 564 consecutive patients underwent SM at our institution. Of these patients, 441 had myocardial resections combined with aortic valve surgery for aortic stenosis and were therefore excluded for the current analysis. Eight patients with HOCM were excluded due to absence of data. We analyzed the remaining 115 patients that underwent surgical intervention for LVOTO due to HOCM or HOCM with concomitant procedures such as MV surgery, aortic valve surgery or coronary artery bypass grafting (CABG).
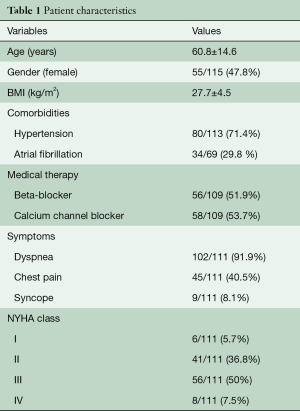
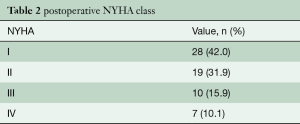
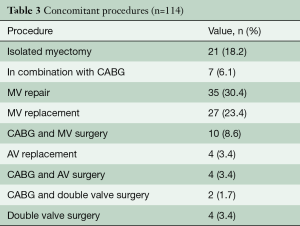
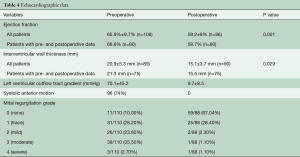
Table 1 describes patient characteristics, preoperative symptoms and cardiac medications. There were more males that underwent the operation (52.2%), with a mean age of 60.8±14.6 years and a body mass index of 27.7±4.5. About half of the patients were preoperatively treated with beta-blocker or calcium channel antagonists. Nearly all patients suffered from dyspnea (n=102, 93%), whereas chest pain or syncope were reported less frequently (40.5% vs. 8.1%, respectively). The degree of exertional dyspnea ranged from none to New York Heart Association (NYHA) class 4, but most patients had NYHA class 3 symptoms (50%) (Table 2). Arterial hypertension and atrial fibrillation were common comorbidities. Table 3 demonstrates the variety of operations that were performed in conjunction with the myectomy. Only one in five patients underwent isolated myectomy, and the most common concomitant operation was MV surgery (n=71; 61.7%). Table 4 shows pre and postoperative echocardiography data. Due to inconsistent follow-up, complete data sets were not available for all 115 patients. Preoperative data demonstrated that the average patient had a normal ejection fraction (EF) with increased LV thickness, left atrial size and LVOT gradient. There was also a significant degree of MV insufficiency.
Full table
Full table
Full table
Full table
Complete (n=95) or a partial sternotomy (n=20) was done to obtain access to the heart. One patient was converted intraoperatively from partial to complete sternotomy. CPB was used at normothermia or mild hypothermia for all our surgical procedures. Mean CPB time was 113.5±66.9 minutes. Myocardial protection consisted of blood cardioplegia or crystalloid cardioplegia (Bretschneider, Dr Franz Köhler Chemie GmbH, Bensheim, Germany).
Five patients required a second operation. Reasons for this were bleeding in two patients, residual mitral regurgitation in two other patients and AV-dissection in another patient. Postoperative complete heart block requiring permanent pacemaker implantation occurred in 17 patients (14.8%). Eleven of these patients underwent SM and valve surgery, five patients underwent isolated SM and one patient underwent SM and CABG. Two patients had a defibrillator implanted. No intraoperative mortality was recorded, however, 8 (6.9%) patients died during their hospital stay. Apart from one patient, all others underwent combined SM and MV surgery. The cause of death was varied—five patients died from low cardiac output, two patients from sepsis, one from pulmonary hemorrhage. Eight patients did not have a clear cause of death. Long-term follow-up data up to 11.3±0.7 years were available for 114 patients (99.1%). During follow-up 16 patients (14%) died.
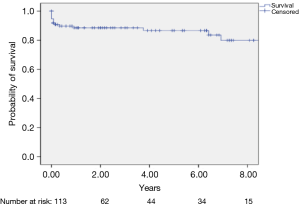
Figure 1 depicts the Kaplan-Meier curve, where the 1, 5 and 8-year survival rates were 90%, 89% and 80%, respectively.
Surgical outcome measured by echocardiography is shown in Table 3. A significant reduction in wall thickness, degree of mitral regurgitation and LVOT gradient are observed. Systolic anterior motion disappeared in 98% of patients.
Discussion
In patients with HOCM, pharmacotherapy is administered to improve functional capacity, reduce symptoms and prevent disease progression (2). If symptoms persist despite medical treatment, SM, alcohol ablation or pacing will then be considered. Medical treatment of symptomatic LVOTO includes non-vasodilating beta-blocker as a first-line agent and disopyramide as a second-line agent. Verapamil can be given in patients who do not tolerate beta-blockers. Low dose thiazide diuretics can also be given to reduce dyspnea, however it should be used cautiously to avoid hypovolemia. Dual-chamber pacing may be considered in addition to medical therapy, however there have been no real benefits reported in regards to clinically relevant endpoints (13,14). TASH has been established over the last years. In experienced centers, TASH has been shown to have similar outcomes to surgical myectomy, with AV-block being the most common complication (7–20%) (15). In 1968, Morrow described SM as a procedure with good postoperative clinical and hemodynamic results (16). SM has been shown to improve quality of life (QoL) and exercise capacity (17). Resection of the septal myocardium alone has been shown to improve mitral regurgitation even when the MV is not operated at the same time (18). Surgical mortality for myectomy with mitral intervention has been reported to be around 3–4% (19).
In this article, we describe the outcomes for surgical myectomy for patients with symptomatic HOCM over the last 20 years at the Leipzig Heart Center. The operative access route and approach varied depending on the extent of the hypertrophied myocardium. Whenever possible we used the minimally invasive approach; however, we were required to convert to a sternotomy in one patient. Our analysis showed that SM is an effective treatment to reduce the LVOTO as well as the degree of mitral regurgitation. Myectomy has previously been shown to improve NYHA functional class (20). The rate of complications in our center has been very low. No iatrogenic aortic regurgitation occurred. Only two patients showed a ventricular septal defect (VSD). Compared to published data our reoperation rate was also low (4.3%) (21).
Mortality has been reported to be as high as 6% in centers with low surgical experience (22). However, increasing surgical expertise has been shown to correlate with a much better survival rates (23). Our intra-hospital mortality was 0.8% for patients with isolated myectomy and 6.1% for patients with myectomy and mitral intervention, which is comparable to published data (24). In-hospital mortality rate has been shown to increase with the complexity of the procedure (21). Furthermore, increased late mortality has been observed in patients who underwent myectomy and additional coronary revascularization or valve replacement (25). On the other hand, studies could demonstrate that the mortality rate after myectomy was similar to a sex- and age-matched general population (20). In our series we had more AV-blocks requiring pacing compared to other centers (26). A possible explanation for this could be the high rate of concomitant operations that were carried out in our center. Schoendube et al. have also noticed this correlation (26). Heric et al. described that patients undergoing pacemaker implantation after myectomy had a similar outcome compared to those who did not require pacing (21).
In conclusion, SM is a safe and effective method to reduce the LVOT gradient in patients with HOCM, and therefore relieve symptoms and increase QoL.
Limitations
Limits of the present study include those inherent to an observational retrospective analysis. As the data was retrieved from our database and not collected as a part of a prospective study, some patients did not have complete follow-up data and were excluded from analysis.
Acknowledgments
We would like to thank Mrs. Funkat from the Leipzig Heart Institute for her help in maintaining the surgical database.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brock R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp Rep 1957;106:221-38. [PubMed]
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Kardiol Pol 2014;72:1054-126. [Crossref] [PubMed]
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381:242-55. [Crossref] [PubMed]
- Olivotto I, Maron MS, Adabag AS, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:480-7. [Crossref] [PubMed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295-303. [Crossref] [PubMed]
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:2761-96. [Crossref] [PubMed]
- Braunwald E, Lambrew CT, Rockoff SD, et al. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation 1964;30 Suppl 4:3-119. [PubMed]
- Maron BJ, Epstein SE, Roberts WC. Causes of sudden death in competitive athletes. J Am Coll Cardiol 1986;7:204-14. [Crossref] [PubMed]
- Ball W, Ivanov J, Rakowski H, et al. Long-term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J Am Coll Cardiol 2011;58:2313-21. [Crossref] [PubMed]
- Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann Surg 1961;154:181-9. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Goodwin JF, Hollman A, Cleland WP, et al. Obstructive cardiomyopathy simulating aortic stenosis. Br Heart J 1960;22:403-14. [Crossref] [PubMed]
- Qintar M, Morad A, Alhawasli H, et al. Pacing for drug-refractory or drug-intolerant hypertrophic cardiomyopathy. Cochrane Database Syst Rev 2012.Cd008523. [PubMed]
- Jeanrenaud X, Goy JJ, Kappenberger L. Effects of dual-chamber pacing in hypertrophic obstructive cardiomyopathy. Lancet 1992;339:1318-23. [Crossref] [PubMed]
- Kuhn H, Lawrenz T, Lieder F, et al. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol 2008;97:234-43. [Crossref] [PubMed]
- Morrow AG, Fogarty TJ, Hannah H 3rd, et al. Operative treatment in idiopathic hypertrophic subaortic stenosis. Techniques, and the results of preoperative and postoperative clinical and hemodynamic assessments. Circulation 1968;37:589-96. [Crossref] [PubMed]
- McCully RB, Nishimura RA, Tajik AJ, et al. Extent of clinical improvement after surgical treatment of hypertrophic obstructive cardiomyopathy. Circulation 1996;94:467-71. [Crossref] [PubMed]
- Yu EH, Omran AS, Wigle ED, et al. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy. J Am Coll Cardiol 2000;36:2219-25. [Crossref] [PubMed]
- Stassano P, Di Tommaso L, Triggiani D, et al. Mitral valve replacement and limited myectomy for hypertrophic obstructive cardiomyopathy: a 25-year follow-up. Tex Heart Inst J 2004;31:137-42. [PubMed]
- Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470-6. [Crossref] [PubMed]
- Heric B, Lytle BW, Miller DP, et al. Surgical management of hypertrophic obstructive cardiomyopathy. Early and late results. J Thorac Cardiovasc Surg 1995;110:195-206; discussion -8.
- Maron BJ, Dearani JA, Ommen SR, et al. The case for surgery in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;44:2044-53. [Crossref] [PubMed]
- Woo A, Williams WG, Choi R, et al. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 2005;111:2033-41. [Crossref] [PubMed]
- Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687-713. [Crossref] [PubMed]
- Sherrid MV, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy: echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg 2003;75:620-32. [Crossref] [PubMed]
- Schoendube FA, Klues HG, Reith S, et al. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation 1995;92:Ii122-7. [Crossref] [PubMed]