Parsimonious assessment for reoperative aortic valve replacement; the deterrent effect of low left ventricular ejection fraction and renal impairment
Introduction
A patient’s underlying comorbidities play a pivotal role in the outcomes of surgical aortic valve replacement (AVR). Contemporary evaluation of these underlying comorbidities is often made through risk predictive models and mortality risk scores, such as the Society of Thoracic Surgeons Predictive Risk of Mortality (STS-PROM) and the European System for Cardiac Risk Evaluation (EuroSCORE) (1-3). One of the few comorbidities consistently present in these predictive models is the history of a prior sternotomy. In the STS Cardiac Surgery Risk Model (STS-CSRM), reoperative AVR (re-AVR) is associated with 2.1 times the odds of operative mortality (OR: 2.11, 95% CI: 1.78–2.49) (4). However, the aggregated risk of concomitant comorbidities is often underestimated in high-risk populations (5). In these patients, the presence of left ventricular ejection fraction (LVEF) and impaired renal function are consistently associated with worse outcomes (6), with outstanding discriminatory power (7).
Low preoperative LVEF is independently associated with worse outcomes after AVR and is a proxy for the patient’s underlying ventricular contractility, an important component in low-gradient aortic stenosis (8,9). In the STS-CSRM, every 10 unit decrease in LVEF is associated with 1.09 times the odds of operative mortality (OR: 1.09, 95% CI: 1.05–1.14) (4).
Impaired renal function, even when mild, has been associated with increased morbidity and mortality after cardiac surgery (6). Severe renal impairment, defined as dialysis dependence, result in 2.85 times the odds of operative mortality according to the STS-CSRM (OR: 2.85, 95% CI: 2.35–3.45) (4).
Patients undergoing re-AVR who present with low LVEF and concomitant renal impairment represent a unique population whose actual risk of mortality may be underestimated by population-based risk models, given their limited accuracy in high-risk populations (5). The use of select highly-predictive baseline characteristics to assess the surgical risk of these patients has shown encouraging results (7). Our aim was to compare the outcomes of re-AVR patients, stratified by their underlying LVEF and assess its interrelation with concomitant renal impairment. We hypothesized that re-operative AVR patients with low preoperative LVEF and impaired renal function represent a prohibitively high-risk population with poor long-term outcomes, therefore providing a parsimonious risk estimation for patients that may benefit from transcatheter therapies.
Methods
Patients and data collection
With permission from the Partners Institutional Review Board, we identified 232 patients ages 18 to 80 years with a previous sternotomy who underwent isolated, re-AVR, between January 2002 and March 2013 at the Brigham and Women’s Hospital. Patients older than 80 years were excluded to reduce possible confounding introduced by unmeasured frailty in this age group. All re-AVR patients were further stratified into groups: “Low” LVEF ≤35% (LEF), n=37 and “High” LVEF >35% (HEF), n=195. Patient characteristics, perioperative data, laboratory test results and in-hospital outcomes were recorded at the time of presentation. Data were extracted from hospital electronic medical records and defined according to the STS Adult Cardiac database version 2.52 unless otherwise noted. STS-PROM were calculated using the 2008 algorithm. Patients were considered to have renal insufficiency if they had a documented history of renal failure or a preoperative creatinine >2.0 mg/dL. Operative mortality was defined as any death occurring in-house during the index admission, or within 30 days of surgery, if discharged. Long-term survival data were obtained from our internal research data repository, routine patient follow-up, and our state Department of Public Health. Follow-up time was calculated in months from the date of surgery to the date of death or May 31, 2014, and censored at last known clinical contact. There was a 99% follow-up for patient survival and the mean follow-up time was 56.8±37.7 months, for a total of 1,117 patient years. Primary outcomes of interest were operative mortality and long-term survival. Secondary outcomes included operative morbidity and length of stay.
Statistical analyses
Normally distributed continuous variables are expressed as mean and standard deviation and were compared using Student’s t-test with Levene’s test for homogeneity of variance. Non-normally distributed variables are expressed as median and interquartile range (IQR) and were compared using Mann-Whitney U tests. Categorical variables are presented as frequencies and percentages and compared using χ2 or Fisher’s exact tests. Longitudinal survival was estimated by Kaplan-Meier analyses. A sparse Cox proportional hazards model was used to evaluate the adjusted risk of low LVEF and renal insufficiency on long-term survival and to test for interactions between them. LVEF and renal insufficiency were selected based on their association with cumulative unadjusted survival and clinical relevance in the scientific literature which mirrored their performance in our unadjusted survival analysis. Age and gender were non-contributory in the survival analysis and were therefore excluded from the final model. All analyses were conducted using IBM SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA) and P≤0.05 was the criterion for significance.
Results
Baseline and operative characteristics
The baseline characteristics of all the reoperative re-AVR cases are described in Table 1. The overall mean age was 68.4±11.5 years and was similarly distributed between LEF (70.0±11.7 years) and HEF (68.1±11.4 years, P=0.351). Patients in the LEF group were more likely to be women (86.5% versus 64.1%, P=0.007), and had a higher frequency of preoperative renal insufficiency (27% versus 5.6%, P=0.001). The prevalence of NHYA class III/IV and congestive heart failure was (as expected) higher for patients in the LEF group (73% versus 44.6%, P=0.002 and 75.7% versus 54.4%, P=0.018, respectively).
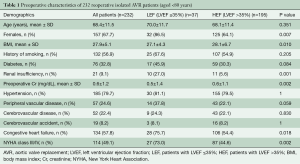
Full table
The most common previous surgeries were isolated coronary artery bypass graft in 53.9% patients, followed by isolated AVR in 22.4% and were not significantly different between the two groups (Table 2). The median interval time from the initial sternotomy to the re-AVR was 9.8 (IQR 6.3–13.5) years in the LEF group and 9.1 (IQR 5.8–12.4) years in the HEF group (P≤0.644). There were 25 patients who underwent a second reoperation; only 4 of them were in the LEF group.

Full table
The most common etiologies behind the re-AVR, calcific or bicuspid native valve disease, were present in 75.9% of the patients (89.2% in the LEF group versus 73.3% in the HEF group, P=0.038). Only 6 patients presented with active endocarditis, all in the HEF group. Structural valve degeneration was present in 14.2% of the patients (8.1% in the LEF and 15.4% in the HEF group, P=0.312). Most re-AVR cases (84.5%) had severe aortic stenosis but only 8.2% had concomitant severe aortic insufficiency, with a similar distribution between the two groups (P=0.23). A detailed description of the etiology and indication behind these reoperative cases is shown in Table 3.
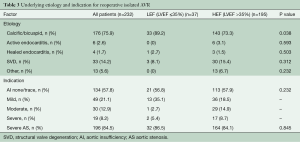
Full table
The cohort’s echocardiographic data are shown in Table 4. As expected, the LEF group had lower mean and peak aortic valve gradients (35.1±15 versus 44.6±17.5 mmHg, P=0.011 and 57.5±18.5 versus 74.6±27.1 mmHg, P=0.001, respectively). Similarly, the left ventricular end-systolic and end-diastolic diameters were higher in the LEF group (4.8±1.8 versus 4.3±1.3 cm, P=0.038 and 4.9±0.7 versus 3.8±1.1 cm, P=0.002, respectively). The mean AV area was smaller than 1 cm2 in both groups (0.7±0.2 in LEF and 0.8±0.2 cm in HEF group, P=0.195).
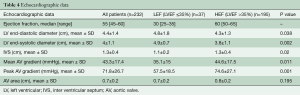
Full table
The majority (72.8%) of the implanted valves were bioprosthetic and were similarly distributed between the LEF and HEF (78.4% versus 71.8%, P<0.55). Despite similar cross-clamp times [84 (IQR 74–125) for LEF and 80 (IQR 66–114) for HEF, P=0.685], patients in the LEF group had significantly longer median perfusion times [150 (IQR 136–237) min versus 143 (IQR 120–197) min, P=0.046]. The use of intraoperative intra-aortic balloon pump (IABP) was higher in patients with LEF (18.9% versus 4.6%, P=0.006).
Operative outcomes
Overall, operative mortality was 4.7% and was significantly higher in the LEF group (13.5% versus 3.1%, P=0.018). Additionally, LEF patients had significantly longer ventilation time [15 (IQR 7–36) h versus 9 (IQR 5–18) h, P=0.026], ICU [90 (IQR 55–167) h versus 51 (IQR 28–115) h, P=0.003] and hospital length of stay [12 (IQR 8–16) days versus 7 (IQR 6–12) days, P=0.001]. There were no differences in the use of postoperative IABP, reoperation for bleeding, and new onset renal insufficiency (Table 5). Although not statistically significant, postoperative stroke (8.1% versus 4.1%, P=0.398) and dialysis (8.1% versus1.5%, P=0.053) were higher in the LEF group.
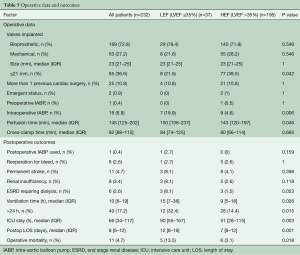
Full table
Survival outcomes
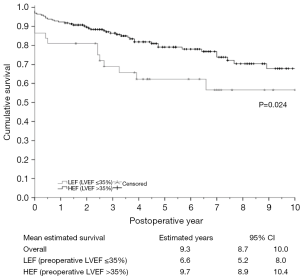
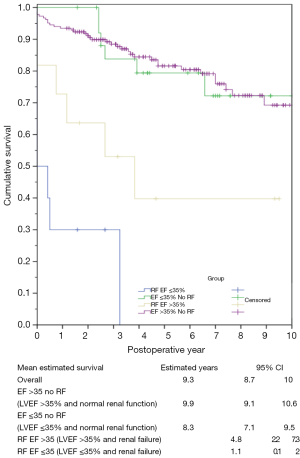
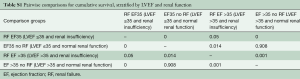
There were 55 deaths during the study period. Long-term survival was significantly lower for LEF, compared to HEF patients (6.6 years, 95% CI: 5.2–8.0 versus 9.7 years, 95% CI: 8.9–10.4, P=0.024) (Figure 1). Unadjusted survival analysis, stratified according to the presence of renal insufficiency, revealed the influence of renal impairment in the survival of LEF and HEF patients. In patients without renal insufficiency, there was no difference in the mean survival between LEF and HEF groups (8.3 years, 95% CI: 7.1–9.5 versus 9.9 years, 95% CI: 9.1–10.6, respectively, P=0.90). Contrary, in patients with renal insufficiency, the mean cumulative survival was significantly lower for patients in the LEF group (1.1 years, 95% CI: 0.1–2.0 versus 4.8 years, 95% CI: 2.2–7.3, P=0.05). Additionally, there was a significant difference between LEF patients with renal insufficiency and without, P=0.001, and HEF patients with renal insufficiency and without, P=0.001 (Figure 2). Pairwise comparisons for cumulative survival, stratified by LVEF and renal function are shown in Table S1.
Full table
Multivariable analysis
In order to determine the adjusted effect of low LVEF and creatinine on long-term survival, we ran a sparse Cox proportional hazards model. In this high-risk population, both creatinine, expressed in 1 mg/dL increments, (HR =4.29 95% CI: 1.830–10.032, P=0.001) and LEF group (HR =5.36, 95% CI: 1.068–26.638, P=0.041) were significant predictors of decreased cumulative survival. In accordance with our unadjusted survival analysis, we observed a significant interaction between LVEF and preoperative creatinine (HR =7.28, 95% CI: 3.120–17.003, P=0.001), explaining the effect of renal impairment across the different levels of LVEF. Age, was non-contributory in our analysis and was therefore not included in the final model.
Discussion
Our study has several noteworthy findings. Patients undergoing re-AVR with low LVEF have a significantly higher operative mortality and longer ventilation, ICU, and hospitalization times. Unadjusted long-term survival was also lower in patients with low LVEF. Interestingly, when stratified by renal function, we observed an unfavorable survival difference for patients with low LVEF in patients with renal insufficiency (preoperative creatinine >2.0 mg/dL) but not in patients with normal renal function. This finding was further confirmed in our adjusted survival analysis, which revealed that low LVEF (<35%) and renal impairment were significant predictors for cumulative survival. Expectedly, we found an interaction between high and low LVEF and renal function, explaining the changing effect of renal function across different levels of LVEF. The prohibitively low cumulative survival seen in re-AVR patients with low LVEF and concomitant renal insufficiency underscores the importance of identifying significant interactions in the patients’ underlying comorbidities and provides a parsimonious approach to recognize these high-risk patients, who are likely to benefit from transcatheter AVR (TAVR).
A 68-year-old (mean age in our study) 174 cm, 70 kg, re-AVR male patient with low LVEF (35%), creatinine of 2.1 mg/dL (renal impairment) and no other comorbidity would have a 2.55% STS-PROM. This is below the intermediate risk threshold (3.0%), for which TAVR is currently indicated, despite the high mortality and low survival observed in LEF patients with concomitant renal insufficiency.
Our study confirms the results from previous publications on isolated re-AVR in which early mortality and decreased long-term survival have been associated with low LVEF and NYHA III/IV (8-12). The steep operative mortality in the LEF group (13.5%) was considerably higher compared to the published mortality of non-reoperative (first time) cardiac surgery patients with low LVEF (5.6% to 11%) (13-15) and to the previously published operative mortality in all re-AVR patients (4.5% to 5.1%) (8,9), to which the 3.2% mortality of our re-AVR HEF group compares favorably. The contrasting outcomes of re-AVR LEF patients to both HEF and historic groups stress the increased risk conferred by the presence of low ventricular contractility and a prior sternotomy.
Long-term survival was significantly shorter in LEF patients. Interestingly, after further stratifying the two groups by renal insufficiency, the interplay between these two variables became evident. The best survival was observed among reoperative AVR patients with no renal insufficiency regardless of their LVEF status, followed by all patients with renal insufficiency in whom the presence of low LVEF resulted in a grim mean survival of 1.1 years. Consequently, an unadjusted difference in the survival function was observed for decreased LVEF across different levels of renal function.
We assess the interrelation between LVEF, renal impairment, and long-term survival using a sparse Cox proportional hazards model. Both low LVEF and renal impairment were significant predictors of cumulative survival. More importantly, we confirmed the presence of a significant interaction between these two variables. Calculating for different clinical scenarios, and compared to HEF patients without renal impairment, HEF patients with renal impairment would have 4.28 times the risk of death (calculated HR =4.28). Correspondingly, for a patient in the LEF group with concomitant renal impairment that risk increases 166.49 times (calculated HR =166.49). The detrimental influence of decreased LVEF and impaired renal function on survival has been previously described (6,16,17). However, in re-AVR patients, the aggregated mortality risk of these comorbidities probably supersedes the predicted risk associated with each independent risk factor.
LVEF is a surrogate marker of cardiac decompensation and deteriorating hemodynamic reserve. Interestingly, the long-term influence of this low LVEF was negligent in patients with normal renal function, and augmented in those with concomitant renal insufficiency. However, the concomitant presence of these three characteristics (reoperative status, low LVEF and renal insufficiency) should serve as a parsimonious warning to the poor outcomes observed in these patients.
Although TAVR has become a popularized intervention for high-risk patients, it lacks the necessary long-term follow-up data (18-21). Greason and colleagues published the outcomes of a high-risk subgroup of reoperative patients from the PARTNER trial (cohort-A), which did not show a significant mortality difference or a conclusive survival benefit with TAVR over surgical AVR (20). More recently, Lauten and associates studied the outcomes of TAVR in patients with low ejection fraction through a sample of low-flow, low-gradient aortic stenosis from the multicenter German TAVI registry (22). Low grade patients suffered higher operative mortality (12.8%) compared to high grade patients (7.4%), although, low grade survivors experienced significant symptomatic improvements at 30 days and 1-year follow-up. These results call for continuous identification of reoperative and low LVEF cases which, based on their underlying comorbidities, would benefit the most from TAVR. Especially in patients with prohibitive reoperative risk, TAVR could offer more than eliminating the risk surgical chest reentry.
Limitations
This study has all the inherent limitations of a retrospective design. The small size of the LEF group precluded further subgroup analysis or a continuous evaluation of LVEF. We decided to adopt LVEF ≤35% as a definition for low LVEF to be aligned with previous publications (23,24). Data on the inotropic reserve of low LVEF patients would help elucidate its role in the observed outcomes and its association with other baseline comorbidities. Unfortunately, Dobutamine stress results were seldom available in our study. However, the underlying inotropic reserve does not predict ventricular recovery in patients who survive the AVR (25), and could be less important in the assessment of long-term outcomes.
Despite recent publications with excellent outcomes of re-AVR in the elderly (10,26), age is still incorporated in the major cardiac surgery risk scores as a significant predictor of postoperative adverse outcomes (3,27,28). The exclusion of patients older than 80 aimed to decreased the variability introduced by unmeasured frailty in the elderly. However, it limits the generalizability of our findings to populations with a similar age distribution. The results of this study should be interpreted with these considerations in mind.
Conclusions
Patients presenting for an isolated re-AVR are a complex and growing population. In our study, re-AVR patients with LVEF <35% experienced higher mortality and longer ventilation, ICU, and hospitalization time, compared to those with higher ejection fraction. Long-term outcomes were heavily influenced by the concomitant presence of renal impairment and low LVEF. Patients undergoing re-AVR who have low LVEF and renal insufficiency represent a prohibitively high-risk population who might benefit from transcatheter therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parsonnet V, Dean D, Bernstein AD. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation 1989;79:I3-12. [PubMed]
- Higgins TL, Estafanous FG, Loop FD, et al. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. Jama 1992;267:2344-8. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2—Isolated Valve Surgery. The Annals of Thoracic Surgery 2009;88:S23-42. [Crossref] [PubMed]
- Shanmugam G, West M, Berg G. Additive and logistic EuroSCORE performance in high risk patients. Interact Cardiovasc Thorac Surg 2005;4:299-303. [Crossref] [PubMed]
- Zakeri R, Freemantle N, Barnett V, et al. Relation between mild renal dysfunction and outcomes after coronary artery bypass grafting. Circulation 2005;112:I270-5. [PubMed]
- Ranucci M, Castelvecchio S, Menicanti L, et al. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation 2009;119:3053-61. [Crossref] [PubMed]
- Leontyev S, Borger MA, Davierwala P, et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg 2011;91:1120-6. [Crossref] [PubMed]
- Onorati F, Biancari F, De Feo M, et al. Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiativedagger. Eur J Cardiothorac Surg 2015;47:269-80; discussion 280. [Crossref] [PubMed]
- Pechlivanidis K, Onorati F, Petrilli G, et al. In which patients is transcatheter aortic valve replacement potentially better indicated than surgery for redo aortic valve disease? Long-term results of a 10-year surgical experience. J Thorac Cardiovasc Surg 2014;148:500-8.e1. [Crossref] [PubMed]
- Kumar P, Athanasiou T, Ali A, et al. Re-do aortic valve replacement: does a previous homograft influence the operative outcome? J Heart Valve Dis 2004;13:904-12; discussion 912-3. [PubMed]
- Fukunaga N, Okada Y, Konishi Y, et al. Clinical outcomes of redo valvular operations: a 20-year experience. Ann Thorac Surg 2012;94:2011-6. [Crossref] [PubMed]
- Carr JA, Haithcock BE, Paone G, et al. Long-term outcome after coronary artery bypass grafting in patients with severe left ventricular dysfunction. Ann Thorac Surg 2002;74:1531-6. [Crossref] [PubMed]
- Ahmed WA, Tully PJ, Baker RA, et al. Survival after isolated coronary artery bypass grafting in patients with severe left ventricular dysfunction. Ann Thorac Surg 2009;87:1106-12. [Crossref] [PubMed]
- Lee S, Chang BC, Yoo KJ, et al. Clinical results of coronary revascularization in left ventricular dysfunction. Circ J 2007;71:1862-6. [Crossref] [PubMed]
- Devbhandari MP, Duncan AJ, Grayson AD, et al. Effect of risk-adjusted, non-dialysis-dependent renal dysfunction on mortality and morbidity following coronary artery bypass surgery: a multi-centre study. Eur J Cardiothorac Surg 2006;29:964-70. [Crossref] [PubMed]
- Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002;106:2687-93. [Crossref] [PubMed]
- Nguyen TC, Babaliaros VC, Razavi SA, et al. Transcatheter aortic valve replacement has improved short-term but similar midterm outcomes in isolated aortic valve replacement after prior coronary artery bypass grafting. Ann Thorac Surg 2014;98:1316-24. [Crossref] [PubMed]
- Papadopoulos N, Schiller N, Fichtlscherer S, et al. Propensity matched analysis of longterm outcomes following transcatheter based aortic valve implantation versus classic aortic valve replacement in patients with previous cardiac surgery. J Cardiothorac Surg 2014;9:99. [Crossref] [PubMed]
- Greason KL, Mathew V, Suri RM, et al. Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass graft operation: a PARTNER trial subgroup analysis. Ann Thorac Surg 2014;98:1-7; discussion 7-8. [Crossref] [PubMed]
- Scherner M, Madershahian N, Kuhr K, et al. Aortic valve replacement after previous heart surgery in high-risk patients: transapical aortic valve implantation versus conventional aortic valve replacement-a risk-adjusted and propensity score-based analysis. J Thorac Cardiovasc Surg 2014;148:90-7. [Crossref] [PubMed]
- Lauten A, Zahn R, Horack M, et al. Transcatheter aortic valve implantation in patients with low-flow, low-gradient aortic stenosis. JACC Cardiovasc Interv 2012;5:552-9. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [Crossref] [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [Crossref] [PubMed]
- Quere JP, Monin JL, Levy F, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006;113:1738-44. [Crossref] [PubMed]
- Onorati F, Biancari F, De Feo M, et al. Outcome of redo surgical aortic valve replacement in patients 80 years and older: results from the Multicenter RECORD Initiative. Ann Thorac Surg 2014;97:537-43. [Crossref] [PubMed]
- Shahian DM, He X, Jacobs JP, et al. The Society of Thoracic Surgeons Isolated Aortic Valve Replacement (AVR) Composite Score: a report of the STS Quality Measurement Task Force. Ann Thorac Surg 2012;94:2166-71. [Crossref] [PubMed]
- Luciani N, Nasso G, Anselmi A, et al. Repeat valvular operations: bench optimization of conventional surgery. Ann Thorac Surg 2006;81:1279-83. [Crossref] [PubMed]





