Outcomes and survival following heart retransplantation for cardiac allograft failure: a systematic review and meta-analysis
Introduction
Orthotopic heart å (HTx) remains the gold standard treatment for end-stage heart disease (1). The International Society for Heart Lung Transplantation (ISHLT) estimates that over 100,000 heart transplants have been performed worldwide (2). With the advancement in immunosuppressive agents, heart preservation techniques, surgical techniques, donor and recipient selection and rejection surveillance, survival of primary HTx recipients at 30 days, 1 year and 5 years approach to 90%, 86% and 70%, respectively (3).
With the increasing population of patients who received HTx, there is a steady population of those who develop cardiac allograft failure secondary to acute rejection, primary graft failure and transplant coronary artery vasculopathy. Several therapeutic interventions including aggressive immunosuppressive therapy, percutaneous transluminal coronary angioplasty, laser myocardial therapy, coronary artery bypass grafting, valvular repair and temporary and long term mechanical circulatory assist devices have been proposed; however, heart retransplantation (RTx) remains the only viable long-term treatment for end-stage cardiac allograft failure (4-8). Despite annual RTx rates of as high as 6% as reported by the 2017 ISHLT data (9), the literature on RTx is ambiguous with several studies reporting conflicting findings in regards to the survival and viability of this therapy (10,11).
Long-term efficacy of RTx remains unclear given the limited worldwide experience and is an important question to elucidate given the shortage of donor organs. The aim of this systematic review was to examine the outcomes of RTx in patients with cardiac allograft failure.
Methods
Literature search strategy
Thorough electronic searches were performed in August 2017 using Ovid Medline, Embase, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), Web of Science, Scopus and CINAHL. To achieve the maximum sensitivity of the search strategy, we combined the terms: “heart retransplantation”, “cardiac retransplantation”, “reoperation”, “graft failure” and “graft survival” as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible studies for the present systematic review and meta-analysis included those that addressed heart RTx amongst HTx recipients. Articles were excluded if they did not contain information about post heart RTx outcomes and survival. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment with no overlapping time intervals. We excluded studies on patients <18 years of age, studies not published in the English language and those not involving human subjects. Furthermore, abstracts, case reports, conference presentations, editorials, reviews and expert opinions were also excluded.
Data extraction and critical appraisal
Data was extracted from article texts, tables and figures (JH Choi, JG Luc). Discrepancies between the two reviewers were resolved by discussion and consensus.
Statistical analysis
A meta-analysis of proportions was conducted for the available main perioperative and postoperative variables with logit transformation. Heterogeneity was evaluated using Cochran Q and I2 test. Meta-regression was conducted using HTx and RTx as subgrouping variables. All analyses were performed using the metafor package for R version 3.01. P values <0.05 were considered statistically significant.
Results
Study characteristics
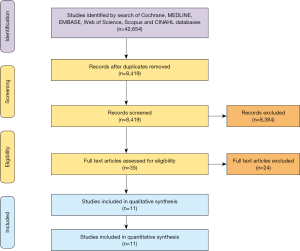
Overall, 8,419 records were identified in the literature search from 1968 to 2011. Following application of the inclusion and exclusion criteria, 11 studies were included for analysis, with a total of 7,791 patients out of which 7,446 patients underwent primary HTx and 345 patients underwent RTx. A PRISMA flow diagram depicting the overall search strategy is provided in Figure 1. Manual search of references did not yield further studies. All studies included in the review were single-center retrospective studies.
Baseline demographics
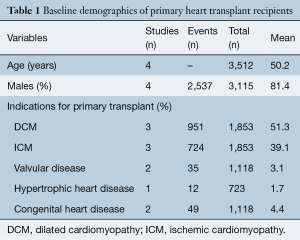
Baseline demographic of recipients undergoing primary HTx and RTx are shown in Tables 1,2, respectively. Mean age of patients undergoing primary HTx and RTx was 50.2 and 49.0 years, respectively, with >81% of patients being male in both groups. The indications for HTx include dilated cardiomyopathy (51.3%), ischemic cardiomyopathy (39.1%), congenital heart disease (4.4%), valvular cardiomyopathy (3.1%) and hypertrophic cardiomyopathy (1.7%).
Full table
Full table
RTx
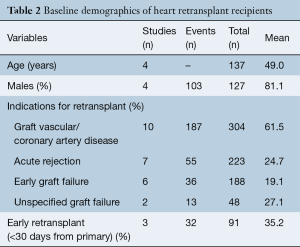
The indications for RTx include allograft vasculopathy (61.5%), acute rejection (24.7%), early graft failure (19.1%). Graft failure etiology was unspecified in 27.1%. In total, 35.2% of patients received RTx within 30 days of getting primary HTx. Mean time interval between primary HTx to RTx was 5.03 years (95% CI: 3.13–6.94).
Primary endpoint: post-transplant survival
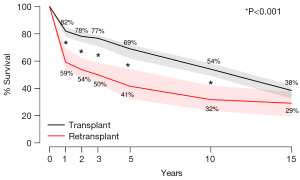
Actuarial survival was significantly higher among HTx patients when compared to RTx patients at 1 year (HTx 81.8% vs. RTx 59.1%, P<0.001), 2 years (HTx 77.9% vs. RTx 53.6%, P<0.001), 3 years (HTx 76.1% vs. RTx 49.8%, P<0.001), 5 years (HTx 68.8% vs. RTx 41.4%, P<0.001) and 10 years (HTx 53.9% vs. RTx 31.7%, P<0.001). This was principally due to a high early mortality of amongst RTx patients (RTx 28.2% vs. HTx 11.2%, P<0.001). Differences in survival were no longer statistically significant between HTx and RTx patients when the survival timepoint was extended to 15 years (Figure 2).
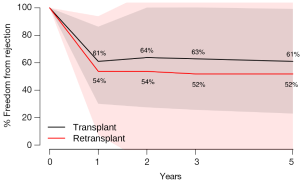
Secondary endpoint: freedom from rejection
There were no significant differences between HTx and RTx in terms of freedom from rejection at 1 year (HTx 61.0% vs. RTx 53.7%, P=0.43), 2 years (HTx 63.8% vs. RTx 53.7%, P=0.26), 3 years (HTx 62.9% vs. RTx 51.9%, P=0.30) and 5 years (HTx 61.0% vs. RTx 51.9%, P=0.36) (Figure 3).
Discussion
Although outcomes following orthotopic HTx have improved, allograft loss is a problem ultimately confronted by many recipients. Although different surgical and medical options have been proposed to overcome allograft failure such as revascularization, valvular repair, mechanical assistance and new pharmacological regimens (8,12-15), heart RTx remains the only definitive management for these patients. Previous studies have demonstrated that outcomes following RTx are inferior to those for primary HTx (10,16-25). Given the limited number of available donor hearts, the long-term results of this treatment option need to be evaluated.
In this systematic review and meta-analysis, the three major indications for heart RTx (coronary allograft vasculopathy, early graft failure and acute rejection) mirror those seen in other heart RTx experiences (20,23). We demonstrate that patients who underwent RTx had a significantly lower survival than those who only underwent primary HTx, principally due to a high early mortality amongst RTx patients. There were no significant differences between HTx and RTx patients in terms of freedom from rejection.
In an analysis of 364 heart RTx reported to the Scientific Registry of Transplant Recipients from 2000 to 2005 (18), 1-, 3- and 5-year unadjusted graft survival was lower in RTx than primary HTx (82% vs. 86%, 70% vs. 80% and 58% vs. 73%, respectively, all P<0.001). However, following adjustment for donor and recipient factors, the relative risk (RR) for graft loss after RTx were comparable to primary HTx at 1-year (RR, 1.34; P=0.151) and 3-years (RR, 1.16; P=0.426) after transplantation. In an analysis of the ISHLT Registry of 1,125 RTx from 1982 to 2003, survival at 1, 3, 5 and 10 years was significantly lower for RTx compared to HTx. An early study of RTx at 13 centers revealed a 1-year survival of only 60% (17). Another study reporting on the outcomes from the Cardiac Transplant Research Database of 106 RTx recipients also confirm that survival after RTx was inferior to primary HTx with a survival of 56% at 1 year and 38% at 5 years (20).
As survival following heart RTx is inferior to that following primary HTx, it is important for further analysis to determine risk factors for poor outcomes following RTx. A previous analysis of the Joint ISHLT/United Network for Organ Sharing Thoracic Registry suggested that the outcome following RTx was significantly affected by the time between transplants, where an inter-transplant interval of less than 2 years resulted in a 2-year survival less than 60% (19). Furthermore, an analysis of data in the Cardiac Transplant Research Database showed that survival following RTx were lowest for acute rejection (32% and 8% at 1 and 5 years, respectively) and early graft failure (50% and 39% at 1 and 5 years, respectively) (20). In another report from Columbia University, the authors reported that since 1993, when selection criteria for RTx excluded those with primary allograft failure and intractable acute rejection occurring less than 6 months after HTx, 1-, 2- and 4-year survival following RTx was 94%, 94% and 94%, respectively (24). These findings suggest that if appropriate candidates are selected for RTx, outcomes can approximate those following primary HTx.
Our study is consistent with the findings of previous studies (10,16-25) though it reports a lower survival than the international average of RTx survival. Reasons for this discrepancy may be due to the high percentage of early (30 days) RTx in our cohort, which has been demonstrated to significantly predict mortality (19). Other reasons include the lack of detail to allow for differentiation of patients based on etiology of allograft failure, inter-transplant interval, era of RTx and other comorbidities such as pre-operative dependence on ventilation or mechanical circulatory support, factors of which have been shown to affect RTx survival. By highlighting inferior early survival of RTx compared to HTx with similar rates of long-term freedom from rejection, our study raises the question as to why outcomes following RTx are inferior to HTx. As seen in Figure 2, the survival curves diverge early in the first year following transplant and then are nearly parallel. This suggests that the differences in survival likely represent perioperative and early postoperative complications such as multi-organ failure, bleeding, and infection, which deserve further exploration (26,27). If the problem of early mortality can be overcome, long-term survival of heart RTx appears to be good.
Since its first clinical application in 1977 (28), the discussion about the justification of heart RTx in the context of donor organ shortage is still ongoing. The Working Group on Heart Retransplantation has published a consensus statement regarding the indications for heart RTx (26). In the consensus statement, Johnson et al. suggest that RTx should be considered in patients with chronic allograft dysfunction, whereas patients with acute rejection and early graft dysfunction should be considered contraindications to RTx (26). Patients on mechanical cardiorespiratory support and those with post-transplant lymphoproliferative disorder should undergo careful consideration on an individual basis (26). Acute graft failure following primary HTx is a clinical dilemma. A widely used salvage strategy for acute graft failure is temporary mechanical circulatory support using venoarterial extracorporeal membrane oxygenation or short-term left or right ventricular assist devices as a bridge to recovery or RTx (8). Recent publications have been encouraging by demonstrating that long-term outcomes for HTx recipients with preoperative left ventricular assist devices were comparable to those without (29-31). Careful patient selection and perioperative care is paramount considering the limited allograft resources.
Limitations
This meta-analysis has several key limitations and must be interpreted with care. Regional differences exist in patient and donor selection, listing practices, access to transplantation, center experience, HTx techniques, immunosuppressive regimes, and clinical management of heart failure. We acknowledge that this heterogeneity in study population is a fundamental limitation that cannot be addressed due to the inability to extract sufficient detail from the pooled data. Pooled results of heart RTx spanning 1968 to 2011 may not correctly reflect the advancements made during the last five decades of this procedure. Moreover, the heterogeneity in results precludes broad generalization into prognostic terms. Due to a lack of detail in the data, we were unable to stratify outcomes of RTx based on early versus late allograft failure, transplantation interval and comorbidities, which are known to affect outcomes. Furthermore, etiology of graft failure was unspecified in 27.1% of patients. Despite these limitations, this study systematically assessed the efficacy and safety of heart RTx for cardiac allograft failure and forms a basis for future studies. Additional studies are needed to identify risk factors for poor outcomes following heart RTx to improve patient and donor selection as well as advancement in perioperative care. It is hoped that these will improve the early mortality of patients requiring heart RTx so that their outcomes can approximate those of primary HTx.
Conclusions
The results of our systematic review of 11 studies consisting of 7,446 patients who underwent primary HTx and 345 patients who underwent RTx demonstrates that patients who underwent RTx had a significantly lower survival than those who only went primary HTx. These were principally due to a high perioperative mortality amongst RTx patients. There were no significant differences between HTx and RTx patients in terms of freedom from rejection. Careful patient selection and perioperative care can make heart RTx a viable option.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol 2008;52:587-98. [Crossref] [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:951-64. [Crossref] [PubMed]
- Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant 2007;26:769-81. [Crossref] [PubMed]
- Dzemeshkevich S, Ragimov A, Mikhaylov Y, et al. Plasmapheresis in the treatment of posttransplant cardiomyopathy. Artif Organs 1998;22:197-202. [Crossref] [PubMed]
- Benza RL, Zoghbi GJ, Tallaj J, et al. Palliation of allograft vasculopathy with transluminal angioplasty: a decade of experience. J Am Coll Cardiol 2004;43:1973-81. [Crossref] [PubMed]
- Halle AA 3rd, DiSciascio G, Massin EK, et al. Coronary angioplasty, atherectomy and bypass surgery in cardiac transplant recipients. J Am Coll Cardiol 1995;26:120-8. [Crossref] [PubMed]
- Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant 2006;6:1248-56. [Crossref] [PubMed]
- Phan K, Luc JGY, Xu J, et al. Utilization and Outcomes of Temporary Mechanical Circulatory Support for Graft Dysfunction After Heart Transplantation. ASAIO J 2017;63:695-703. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Schnetzler B, Pavie A, Dorent R, et al. Heart retransplantation: a 23-year single-center clinical experience. Ann Thorac Surg 1998;65:978-83. [Crossref] [PubMed]
- Shuhaiber JH, Kim JB, Hur K, et al. Comparison of survival in primary and repeat heart transplantation from 1987 through 2004 in the United States. Ann Thorac Surg 2007;83:2135-41. [Crossref] [PubMed]
- Musci M, Loebe M, Wellnhofer E, et al. Coronary angioplasty, bypass surgery, and retransplantation in cardiac transplant patients with graft coronary disease. Thorac Cardiovasc Surg 1998;46:268-74. [Crossref] [PubMed]
- Bader FM, Kfoury AG, Gilbert EM, et al. Percutaneous coronary interventions with stents in cardiac transplant recipients. J Heart Lung Transplant 2006;25:298-301. [Crossref] [PubMed]
- Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int 2009;22:78-89. [Crossref] [PubMed]
- Klauss V, König A, Spes C, et al. Cyclosporine versus tacrolimus (FK 506) for prevention of cardiac allograft vasculopathy. Am J Cardiol 2000;85:266-9. [Crossref] [PubMed]
- Karwande SV, Ensley RD, Renlund DG, et al. Cardiac retransplantation: a viable option? The Registry of the International Society for Heart and Lung Transplantation. Ann Thorac Surg 1992;54:840-4; discussion 845. [Crossref] [PubMed]
- Ensley RD, Hunt S, Taylor DO, et al. Predictors of survival after repeat heart transplantation. The Registry of the International Society for Heart and Lung Transplantation, and Contributing Investigators. J Heart Lung Transplant 1992;11:S142-58. [PubMed]
- Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant 2007;7:1424-33. [Crossref] [PubMed]
- Srivastava R, Keck BM, Bennett LE, et al. The results of cardiac retransplantation: an analysis of the Joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. Transplantation 2000;70:606-12. [Crossref] [PubMed]
- Radovancevic B, McGiffin DC, Kobashigawa JA, et al. Retransplantation in 7,290 primary transplant patients: a 10-year multi-institutional study. J Heart Lung Transplant 2003;22:862-8. [Crossref] [PubMed]
- Topkara VK, Dang NC, John R, et al. A decade experience of cardiac retransplantation in adult recipients. J Heart Lung Transplant 2005;24:1745-50. [Crossref] [PubMed]
- Schlechta B, Kocher AA, Ehrlich M, et al. Heart retransplantation: institutional results of a series of 31 cases. Transplant Proc 2001;33:2759-61. [Crossref] [PubMed]
- Smith JA, Ribakove GH, Hunt SA, et al. Heart retransplantation: the 25-year experience at a single institution. J Heart Lung Transplant 1995;14:832-9. [PubMed]
- John R, Chen JM, Weinberg A, et al. Long-term survival after cardiac retransplantation: a twenty-year single-center experience. J Thorac Cardiovasc Surg 1999;117:543-55. [Crossref] [PubMed]
- Mahle WT, Vincent RN, Kanter KR. Cardiac retransplantation in childhood: analysis of data from the United Network for Organ Sharing. J Thorac Cardiovasc Surg 2005;130:542-6. [Crossref] [PubMed]
- Johnson MR, Aaronson KD, Canter CE, et al. Heart retransplantation. Am J Transplant 2007;7:2075-81. [Crossref] [PubMed]
- Tsao L, Uriel N, Leitz K, et al. Higher rate of comorbidities after cardiac retransplantation contributes to decreased survival. J Heart Lung Transplant 2009;28:1072-4. [Crossref] [PubMed]
- Copeland JG, Griepp RB, Bieber CP, et al. Successful retransplantation of the human heart. J Thorac Cardiovasc Surg 1977;73:242-7. [PubMed]
- Pal JD, Piacentino V, Cuevas AD, et al. Impact of left ventricular assist device bridging on posttransplant outcomes. Ann Thorac Surg 2009;88:1457-61; discussion 1461. [Crossref] [PubMed]
- Liden H, Haraldsson A, Ricksten SE, et al. Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? Eur J Cardiothorac Surg 2009;35:1029-34; discussion 1034-5. [Crossref] [PubMed]
- Russo MJ, Hong KN, Davies RR, et al. Posttransplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg 2009;138:1425-32.e1-3.





