Tracheal release maneuvers
Introduction
Tracheal resection and reconstruction has been slow to develop in the field of thoracic surgery. Historically, these cases have been limited to large specialized referral centers. Understanding the historical background of this procedure and maneuvers to safely perform a well-vascularized, low tension anastomosis has allowed advancement of the field of tracheal surgery with excellent outcomes. Historically, tracheal resection was thought to be limited to 2 cm. In 1948, Rob and Bateman published a small series of patients in The British Journal of Surgery describing their experience with tracheal resection (1). They, along with Belsey, described the “2 cm rule” as the extent for tracheal resection with primary reconstruction. Both authors chose synthetic material for reconstruction, typically consisting of tantalum gauze or steel wire with mixed results (1,2). Further characterization of tracheal blood supply and limits of resection based on tension expanded and modernized resections of the trachea.
Extensive arterial mapping studies by Salassa and colleagues as well as Miura and Grillo have defined the blood supply to the trachea (3-5). Contributions of inferior thyroid, subclavian, supreme intercostal, internal mammary, innominate and superior and middle bronchial arteries all contribute to tracheal blood supply, depending on anatomical region. Salassa further described five patterns of arterial blood supply that are important for tracheal resection: (I) lateral longitudinal anastomosis linking segmental vessels; (II) transverse intercartilaginous arteries which link left and right sides; (III) tracheoesophageal arteries; (IV) anastomosis of tracheal vessels with cervical trachea; (V) anastomosis of paracarinal nodal arteries with the distal trachea. Understanding these principles allows the surgeon to appreciate that tracheal blood supply is segmental, lateral blood supply should be preserved, collateral circulation can provide a safety net against tracheal ischemia and skeletonization of proximal and distal ends of anastomosis should be minimized (6).
Preoperative evaluation
The preoperative planning and evaluation of patients undergoing tracheal resection can not be over-emphasized. This includes a detailed history and physical, radiographic imaging of the airway and detailed bronchoscopy. Imaging can include anteroposterior and lateral films of the chest and neck. Computed tomography (CT) is more sensitive and can provide more accurate localization of tracheal disease. CT tracheal protocols can include inspiratory and expiratory phases to evaluate dynamic obstruction when tracheomalacia may be suspected. If the tracheal resection is being done for neoplastic reasons, CT of the neck and chest are mandatory as it can detect locally advanced or metastatic disease and the addition of intravenous contrast can be beneficial for evaluating proximity to neck and mediastinal vasculature.
Bronchoscopic evaluation of the trachea is essential to preoperative planning. We prefer the rigid bronchoscope as it is not only diagnostic, but can be used for therapeutic dilations. The bronchoscope should be used to make critical measurements and determine whether the lesion is resectable. The measurements from vocal cords to the lesion, actual length of lesion and distance from lesion to carina are necessary not only to determine location and length of trachea needed to be resected, but can give the surgeon insight toward the need for tracheal release maneuvers.
Tracheal release maneuvers are not required for every tracheal resection; as discussed below, some simple maneuvers can be performed in every case that will minimize anastomotic tension. With normal neck flexion, there is caudal displacement of the trachea. In cadaveric experiments, Mulliken and Grillo showed that with 15° to 35° of neck flexion, a 4.5 cm length of trachea can be resected with a tension-free primary anastomosis (7). Individual body habitus, range of neck motion and less cervical mobility in those with previous cervical pathology or patients with advanced age should be considered. The neck is initially in an extended position to deliver as much of the trachea into the neck while the tracheal resection is performed. Once all anastomotic stitches are placed, the neck is then placed in a flexed position while sutures are tied down. A “guardian stitch” is placed in the midline from the submental crease to the anterior chest at about the angle of Louis. The stitch is loosely tied and should not pull the chin down on tension. Its purpose is to remind the patient to maintain neck flexion. The avascular pretracheal plane is bluntly dissected, taking special care to avoid injury to the lateral blood supply of the trachea. If performing a cervical resection, this can be performed through the collar incision. If performing an intrathoracic tracheal resection, a mediastinoscopy can be performed first with a standard low cervical incision. This is then closed and the patient placed in lateral decubitus position for a standard thoracotomy approach. It is worth noting that mediastinoscopy performed as a separate advanced procedure to tracheal or carinal resection may cause tracheal adhesions which makes for a more difficult dissection at the time of resection. Neck flexion and dissection of the pretracheal plane are routinely used and typically provide enough relief of anastomotic tension for most tracheal resections (6).
Laryngeal and suprahyoid release maneuvers
Release maneuvers of the larynx and hyoid bone may be beneficial in the setting of extended resection of the trachea in upper and mid-tracheal lesion. In our experience, it is rarely needed and provides very little length in distal or intrathoracic resections (8). It should be noted that laryngeal release should not be routinely used as it is not without potential risk (9). Dedo and Fishman described the first laryngeal release between the thyroid cartilage and the thyrohyoid muscle/membrane (10). This technique, while effective, was complicated by consistent postoperative dysphagia and aspiration. This typically improved, but may take up to 6 months. Similar results were seen in the Toronto General Hospital series. Our institution does not use this technique. Dedo himself moved away from this technique in his text Surgery of the Larynx and Trachea, where he describes the suprahyoid technique which gives equivalent length without the risk to the superior laryngeal artery/vein and internal nerve branch (11). The suprahyoid laryngeal release maneuver developed by Montgomery in 1974 has decreased the incidence of laryngeal dysfunction and is the maneuver of choice at our institution (12). The overall length gained by suprahyoid release is 1 to 2 cm and is independent of length gained with neck flexion.
Technical considerations
Thyrohyoid laryngeal release (Dedo)
In this maneuver, the larynx is allowed to drop by detaching the thyrohyoid muscles with the thyrohyoid membrane. The sternohyoid and omohyoid muscles are then retracted laterally. The superior cornua of the thyroid cartilage is then detached on each side and the larynx is able to be displaced caudally. Special care should be made to not injure the internal branches of the superior laryngeal nerve, which runs posterior and medial to the superior cornua of the thyroid cartilage (9).
Suprahyoid release (Montgomery)
A low transverse collar incision is used for tracheal resection and reconstruction. If a laryngeal release is required, we prefer a second, short 4 cm transverse incision directly over the hyoid bone. This avoids an aesthetically unpleasing scar and is typically well hidden in the crease of the upper neck. Dissection is then carried down through the subcutaneous fat and platysma to the hyoid bone. The cephalad surface of the hyoid bone is initially exposed and dissection is carried out laterally to each side with care to preserve the digastric sling on either side. Using cautery, all muscles attached to the hyoid bone between the two digastric slings are divided. This includes mylohyoid, geniohyoid, genioglossus and the tendons of the chondroglossus muscle attaching to the lesser cornua (Figures 1,2). Using heavy Mayo scissors, the hyoid bone is divided on each side lateral to the lesser cornua, but medial to the digastric sling. We typically use a small flat suction drain placed in the preepiglottic space and then close the incision in two layers. It is important to close the incision at this time due to this area of the neck becoming inaccessible when the neck is placed in flexion for the tracheal anastomosis (9,13).
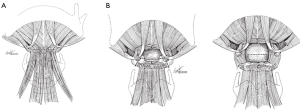
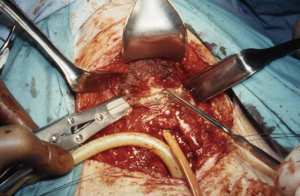
Results
In a large series of patients from our institution undergoing tracheal resection for postintubation stenosis, laryngeal release only occurred in 9% of 521 operations. The incidence of laryngeal release increased in the setting of re-resections occurring in 29% of secondary operations, versus 6.4% of primary operations. The average length of trachea resected with laryngeal release maneuver was 4.4 cm. All patients had either good or satisfactory outcome (14). Other series from our institution show laryngeal release maneuvers were needed in only 8.7% of 80 laryngotracheoplastic resections and in 6% of 108 tracheal resections for primary or secondary tumors (8,14). The most common sequelae of suprahyoid tracheal release is related to dysphagia and aspiration (13). In the postintubation stenosis series published by Grillo, the incidence of laryngeal dysfunction (aspiration or vocal cord dysfunction) after laryngeal release was 5%. Two patients required gastrostomy tube for prolonged aspiration (14).
Hilar and pericardial release maneuvers
Laryngeal release for lower tracheal resections has limited utility. If an extended lower tracheal, carinal or bronchial mainstem resection is performed, a hilar or pericardial release maneuver is of more utility. The possibility of intrathoracic release maneuvers should be considered when planning the operative approach. Most distal tracheal resections are approached via right thoracotomy. This establishes easy access to the pericardium and right hilum for adjunctive release maneuvers. Other methods of access for intrathoracic release maneuvers include VATS, median sternotomy and although rarely indicated bilateral thoracotomy or clamshell approach. If a left hilar release is considered, median sternotomy with a “T” incision through the left fourth interspace can provide good access to the left pleural space. Conversely, it is possible to perform pericardial release within the pericardium. Great care must be exercised to avoid injury to the phrenic nerve.
Technical considerations
Right hilar release
Some mobility can be obtained by blunt dissection of the avascular plane anterior to the left and right mainstem bronchi. Special care must be taken to preserve the bronchial blood supply. This along with division of the inferior pulmonary ligament will add minimal length, but more importantly, will provide excellent access to the pericardium for the more beneficial intrapericardial release discussed below (15,16).
Intrapericardial release
Depending on the need and access, intrapericardial release can be performed on the left, right or bilaterally. As previously stated, for distal tracheal tumors this is typically encountered from the right side and addresses the right hilum/pericardium. Once the inferior pulmonary ligament is divided and the pericardium is cleared posterior to the phrenic nerve, the pericardium is incised inferior to the inferior pulmonary vein. A “U” shaped incision is made in the pericardium that is oriented anterior, inferior and posterior to the inferior pulmonary vein, but posterior to the plane of the phrenic nerve (Figures 3,4). The longitudinal tissue that attaches the pericardium to the epicardium is divided above the entry point of the inferior pulmonary vein into the atrium and below towards the inferior vena cava for 1 to 2 cm. The pericardial incision can be carried out further, completely circumcising the hilum. Injury should be avoided to the phrenic nerve and special care should be made to preserve bronchial blood supply and lymphatics near the main bronchus. Once the anastomosis is performed, the pericardium does not need to be closed (15,16).
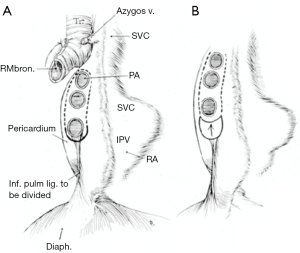
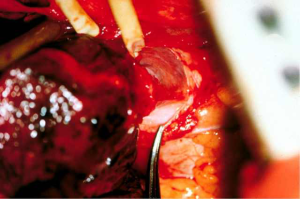
Left hilar and pericardial release can be useful for left main bronchus resections, carinal resection or extensive distal tracheal resection. This can be carried out in similar fashion to the right side with the caveat that left thoracotomy is rarely needed with the exception of distal left main bronchus resections. Access to either hilum can be achieved through median sternotomy. Transpleural approach on the left side, while feasible, is typically not tolerated well due to cardiac retraction. Wright described an intrapericardial approach via a wide anterior pericardiotomy. The pericardium is incised on the inside of the pericardium and must be close to the pulmonary vein as to not injure the phrenic nerve. This is then extended anterior, inferior and posterior to the inferior pulmonary vein as previously described. On the right, pericardial release is typically performed transpleural, as an intrapericardial approach is difficult due to the right atrium and inferior vena cava obstructing visualization (15,16).
Results
Hilar and pericardial release maneuvers are typically well tolerated. Complications can be related to hemorrhage due to proximity of main pulmonary vessels and this risk can be exacerbated by scar tissue and adhesions from previous operations. Risk of phrenic nerve injury should also be considered. Direct visualization during transpleural dissection and staying close to the inferior pulmonary vein during intrapericardial anterior dissection can minimize risk of injury.
Mitchell et al. reviewed 134 total patients undergoing carinal resection at our institution. Hilar release maneuvers were used in 37% of patients. The rate of anastomotic complications for the entire cohort was 17.2% (23 patients). This included necrosis, stenosis, bronchial mucosal sloughing and excessive granulation tissue formation. Ten of these patients died within the first year demonstrating how difficult anastomotic complications can be to manage (17).
Conclusions
The key to a successful tracheal resection is careful preoperative planning. Accurate measurements of the lesion and amount of trachea needing to be resected is critical. This will allow the surgeon to plan operative approach and the potential need for release maneuvers. We have found that with simple neck flexion and pretracheal dissection 4 cm of trachea can be removed without further release maneuvers. The addition of laryngeal and hilar/pericardial maneuvers may allow nearly 50% of the trachea to be resected. The ultimate goal of the operation is to achieve a well-vascularized and low tension anastomosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rob CG, Bateman GH. Reconstruction of the trachea and cervical oesophagus; preliminary report. Br J Surg 1949;37:202-5. [Crossref] [PubMed]
- Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg 1950;38:200-5. [Crossref] [PubMed]
- Dai L, Mick SL, McCrae KR, et al. Preoperative Anemia in Cardiac Operation: Does Hemoglobin Tell the Whole Story? Ann Thorac Surg 2018;105:100-7. [Crossref] [PubMed]
- Miura T, Grillo HC. The contribution of the inferior thyroid artery to the blood supply of the human trachea. Surg Gynecol Obstet 1966;123:99-102. [PubMed]
- Grillo HC. Tracheal blood supply. Ann Thorac Surg 1977;24:99. [Crossref] [PubMed]
- Heitmiller RF. Tracheal release maneuvers. Chest Surg Clin N Am 2003;13:201-10. [Crossref] [PubMed]
- Mulliken JB, Grillo HC. The limits of tracheal resection with primary anastomosis: further anatomical studies in man. J Thorac Cardiovasc Surg 1968;55:418-21. [PubMed]
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77. [Crossref] [PubMed]
- Grillo HC. Tracheal reconstruction: anterior approach and extended resection. In: Grillo HC. editor. Surgery of the Trachea and Bronchi. Hamilton: BC Decker Inc., 2004:539.
- Dedo HH, Fishman NH. Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 1969;78:285-96. [Crossref] [PubMed]
- Dedo HH. Surgery of the Larynx and Trachea. 1st ed. PMPH, USA, 1990.
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [Crossref] [PubMed]
- Nasir BS, Liberman M. Release Maneuvers: Suprahyoid Laryngeal Release. In: Mathisen DJ, Morse CR. editors. Master Techniques in Surgery, Thoracic Surgery: Transplantation, Tracheal Resections, Mediastinal Tumors, Extended Thoracic Resections. Wolters Kluwer, 2015:303-7.
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3. [Crossref] [PubMed]
- Khereba M, Nasir BS, Liberman M. Release Maneuvers: Pericardial Release. In: Mathisen DJ, Morse CR, eds. Master Techniques in Surgery, Thoracic Surgery: Transplantation, Tracheal Resections, Mediastinal Tumors, Extended Thoracic Resections. Wolters Kluwer, 2015:309-13.
- Grillo HC. Reconstruction of the lower trachea (transthoracic) and procedures for extended resection. In: Grillo HC. Surgery of the trachea and bronchi. Hamilton, London: BC Decker Inc., 2004:587-98.
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]





