Idiopathic subglottic stenosis: techniques and results
Introduction
Pathophysiology and diagnosis
Idiopathic subglottic stenosis is a rare condition of unknown etiology. It most commonly affects adult female Caucasian patients and is characterized by a circumferential fibrotic stenosis in the subglottic larynx and upper trachea (1). The proximal extent of the stricture begins at a variable distance from the vocal cords and generally extends 2 to 3 cm, with a range of 0.5 to 5 cm. The point of maximal stenosis usually lies at the cricoid or first tracheal ring.
Patients generally present with symptoms including dyspnea on exertion, cough, wheezing, stridor and difficulty clearing secretions. This pattern of symptoms often presents over months to year and may progress to dyspnea and stridor at rest. The diagnosis of idiopathic subglottic stenosis is made by the clinical characteristics just described and the exclusion of other causes including collagen vascular disease, relapsing polychondritis, scleroderma, polyarteritis, sarcoid and granulomatosis with polyangiitis.
Preoperative assessment and operative indications
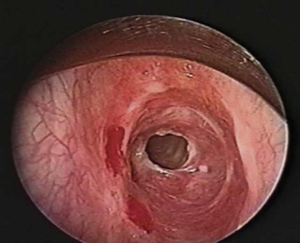
Simple soft tissue radiographs of the neck can identify narrowing in the subglottic space, however high resolution computed axial tomography with 3D reconstruction has become the preferred method of imaging, and allows measurements of the length and configuration of the stenosis as well as its relationship to surrounding structures. Direct bronchoscopy is essential and typically demonstrates a short, concentric stenosis centered at the cricoid (Figure 1). The proximity and severity of the process in relation to the undersurface of the vocal cords and the degree of inflammation is of great importance and should be assessed.
In general, any patient who presents with airway stenosis should be considered for a tracheal resection and reconstruction. Absolute contraindications include a non-reconstructible airway (usually due to an excessive length of damaged airway), severe comorbidities or a prolonged need for mechanical ventilation. Relative contraindications include a history of radiation to the trachea which limits tracheal mobility and impairs microvascular blood supply, active mucosal inflammation of the trachea and active steroid therapy. Steroids should be weaned prior to tracheal reconstruction because they interfere with healing of the tracheal anastomosis. If necessary, tracheal dilation can be delayed until inflammation subsides and steroids are discontinued.
Operative techniques
Preparation
A single-staged laryngotracheal resection and reconstruction is our preferred method of treatment. Anesthesia for this procedure is best administered as total intravenous anesthesia (TIVA). This process provides satisfactory muscle relaxation and blunting of the airway reflexes, decouples ventilation and the delivery of anesthesia, and avoids environmental contamination of volatile anesthetics during the case.
To begin the procedure, the patient is placed supine on the operating table with a roll or inflatable bag underneath the shoulders to extend the neck. Rigid bronchoscopy should be performed to reassess the lesion and the degree of inflammation, proximal involvement, or presence of fibrous bands. Active inflammation or proximal involvement should be dilated only and closely followed with definitive repair delayed. If surgery is to proceed, a stricture less than 6 mm in diameter is gently dilated under direct vision with rigid pediatric bronchoscopes to allow passage of an endotracheal tube. If the stricture is more than 6 mm, a small endotracheal tube (5.5 mm) may be placed through the lesion.
Exposition
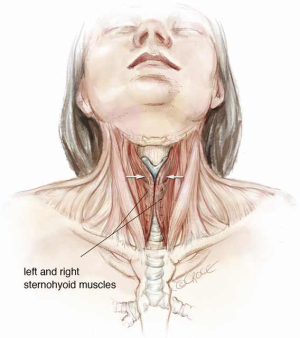
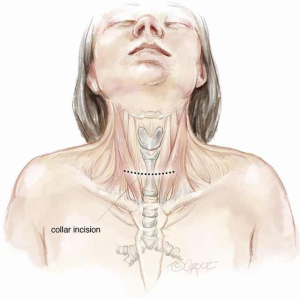
A cervical or upper cervico mediastinal approach is used and the trachea is explored through a collar incision (Figure 2). Skin flaps are raised with platysma to the cricoid cartilage superiorly and the sternal notch inferiorly. The medial margins of the strap muscles are elevated and the anterior surface of the trachea is bluntly exposed from the cricoid cartilage to the carina. The thyroid isthmus is divided, dissected from the trachea, and retracted laterally with sutures (2). It is essential to keep the dissection close to the trachea to avoid injury to the recurrent laryngeal nerves (which enter the larynx just medial to the inferior cornua of the thyroid cartilages), esophagus and back wall of the innominate artery. If the innominate artery is adherent to the trachea and requires dissection, a strap muscle flap should be interposed between the artery and tracheal anastomosis at the completion of reconstruction. The dissection is made meticulously along the lateral borders of the involved trachea and posteriorly approximately 1 cm below the lesion.
Operation
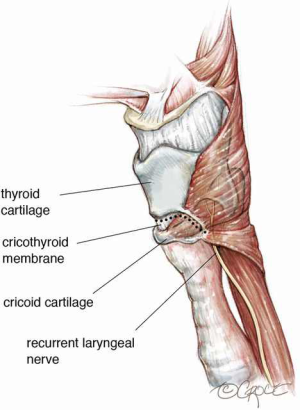
If the stenosis involves only the upper trachea or extends upward only to the lower margin of the cricoid cartilage, standard segmental circumferential tracheal resection is performed with an end-to-end anastomosis. This usually requires anastomosis of the trachea to the inferior margin of the cricoid cartilage. However, if the stenosis involves the subglottic larynx, as is most often the case, resection must be modified to preserve the posterior skeleton of the larynx to protect the entry point of the recurrent laryngeal nerves (3). The nerves are protected by beveling off the cricoid anteriorly and laterally while preserving the posterior plate. The anterior cricoid is then resected with a line of transection through the cricothyroid membrane (Figure 3).
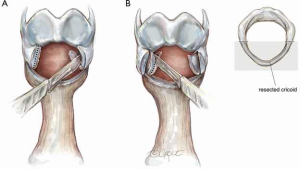
In patients with a very high stenosis and lateral narrowing who are not deemed to have an adequate inner luminal area after standard laryngotracheal resection, a modified ‘tailored cricoplasty’ is performed (4). In this step, a sharp submucosal resection of thick submucosal tissue is performed laterally (Figure 4A). The inner one third to one half of the lateral cricoid cartilage is carefully excised, with particular care taken to preserve the mucosa over the resected cartilage as a posterior pedicled flap (Figure 4B). The exposed cricoid cartilage is then resurfaced by advancing the preserved mucosal flap over the cricoid and suturing it with interrupted 5-0 Vicryl sutures (Figure 5). Sutures are placed in a simple interrupted manner through the mucosal flap, pulling the flap to cover the exposed cartilage and then through the remaining cricoid. Knots are tied on the outside of the airway. Usually only one or two sutures are required. The subsequent anastomotic sutures will also fix the mucosal flap to the cricoid (5).
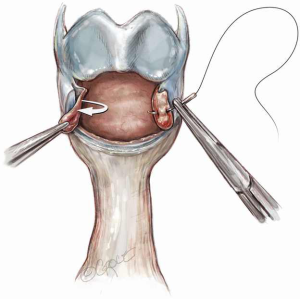
After cricoplasty, the distal normal trachea below the level of the stenosis is beveled for use in reconstruction. The first normal cartilage below the stricture is salvaged and is cut backward in a sloping line toward its posterior ends, to create a ‘prow’ from this single cartilage (Figure 6). The trachea should be appropriately tailored so that the proximal trachea coapts well with the cut edge of the larynx (6). Circumferential dissection of the remaining trachea is limited to no more than 1 cm to protect the segmental blood supply which enters laterally. Devascularization of healthy trachea that will be anastomosed invites possible necrosis and anastomotic dehiscence.

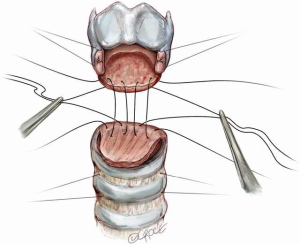
Both proximally and distally, 2-0 Vicryl (Ethicon, Somerville, NJ, USA) traction sutures are placed on either side of the trachea in the midlateral position. These sutures pass vertically through the full thickness of the tracheal wall and around one or more rings. Proximally, they involve the lateral laryngeal wall. Four absorbable sutures (4-0 Vicryl) are then placed in a line across the back of the base of the posterior tracheal wall to the inferior margin of the posterior cricoid plate, to fix the flap against the cartilage (Figure 7). These are vertical mattress sutures on the membranous wall. The sutures are clipped to the drapes on either side.
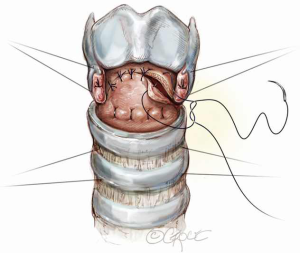
The anastomosis is then created with 5-0 Vicryl sutures which are placed from the posterior mucosa of the larynx to the trachea with knots inverted from the lumen (Figure 8). For ease of placement, these simple sutures can be placed in such a way that the knots are on the inside of the lumen. The sutures are placed but not tied and continued serially until a point is reached at the midlateral tracheal traction suture. Sutures anterior to the location of the traction sutures on either side are then placed. The sutures are placed through the cartilage approximately 4 mm from the cut edge of the trachea and 4 mm apart. The patient’s head is firmly supported on blankets in flexion and the crossed lateral traction sutures are then pulled together on either side and tied with surgeon’s knots opposing the tracheal ends (Figure 9A). Sutures are next tied in the following order: sutures anterior to the traction sutures (Figure 9B), lateral sutures on both sides, and finally the anastomotic sutures posterior to the lateral traction sutures (Figure 9C).
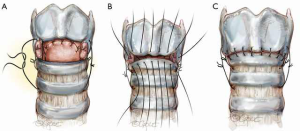
The cut traction sutures are left in place to guard against tension on the anastomotic sutures. The integrity of the anastomosis is checked by submerging it in saline solution, deflating the tube cuff, and insufflating to pressures between 20 and 30 cmH2O. All anastomoses must be air tight and a patient should not leave the OR with an air leak. A leak must be repaired, even if it means taking the anastomosis down and redoing it again. The sternohyoid muscle or the thyroid isthmus is then used to cover all suture lines (Figure 10). This serves as a buttress both as an adjunct to healing and a barrier to superficial wound infections. If there is any concern regarding dissection of the innominate artery, a pedicled strap muscle should be interposed between the innominate artery and the trachea.
If it is determined after neck flexion that excessive anastomotic tension exists, maneuvers should be performed to reduce tension. The most helpful maneuver for the upper and mid-trachea is the Montgomery suprahyoid release (7). This can be performed by exposing the hyoid through a small horizontal incision just over the hyoid. The muscles inserting on the superior aspect of the hyoid between the lesser cornu are divided. The hyoid bone is divided just lateral to the lesser cornu on both sides. This release maneuver usually provides between 1 and 2 cm of additional mobility to the trachea.
Flat suction drains are placed in the pretracheal and substernal spaces, and the strap muscles are approximated in the midline. After the incision is closed, a heavy suture is placed through the submental skin crease beneath the chin and through the presternal skin. This suture is tied with the patient’s neck in moderate flexion to prevent sudden hyperextension of the neck in the first week after the operation.
Completion
After tracheal resection and reconstruction, patients are usually extubated in the operating room. If progressive glottic or subglottic edema occurs postoperatively, a short course of steroids can be given for 24 hours (Decadron 10 mg IV, then 4 mg IV every 6 hours for 3 doses). Useful adjuncts include elevation of the head of the bed, as well as diuretics and fluid restriction and heliox. If this fails, patients may be intubated with a small-bore, uncuffed endotracheal tube. After several days, patients are returned to the operating room for extubation under anesthesia. If the airway is still inadequate, a small tracheostomy tube is carefully placed two rings below the anastomosis (8).
Comments
Clinical results
In the largest series to date, our institution reported the outcomes of 263 patients diagnosed with idiopathic subglottic stenosis and treated with laryngotracheal resection and reconstruction (9). Resections ranged from 1 to 5 cm, averaging 2.6 cm. Of the 263 patients, 243 (93.5%) were extubated in the operating room, one remained intubated for 6 days and 16 (6.1%) required a temporary protective tracheostomy at the end of the operation. These occurred early in our experience and no protective tracheostomies have been placed in the last 190 patients. There were no perioperative deaths. Intensive care unit stays averaged 1.23 days and total hospital length of stay averaged 8.1 days. Surveillance bronchoscopy was routinely performed at 1 week post operatively, and of the 223 patients who underwent bronchoscopy, 38 had prominent vocal cord edema, 15 had supraglottic edema, 17 had subglottic edema and 6 had granulations. Only 24.3% of patients were treated with steroids for airway edema. Thirty-day morbidity included 31 patients (11.8%) with anastomotic complications, including 17 (6.4%) with granulations, 7 (2.6%) with subcutaneous emphysema, 3 (1.1%) with early stenosis and 3 (1.1%) with cartilage necrosis and separation. Only 2 patients (0.7%) had recurrent nerve palsies. In those patients with cartilaginous separation or impending anastomotic separation, urgent intra-operative bronchoscopy was essential to assure the integrity of the airway. If the airway was found to be intact, then treatment was initiated with broad spectrum antibiotics, inhaled tobramycin and, for a select number of patients, hyperbaric oxygen therapy.
Over a median follow-up of 66 months, delayed recurrence, determined by symptoms and bronchoscopic findings, occurred in 23 (8.7%) patients. Of these 23 patients, 14 were classified as having mild recurrence, which was defined as only requiring an occasional dilation with relief of symptoms. Nine were classified as recalcitrant, requiring repeated dilations or laser therapy with a frequency of every 6 months. No patients required reoperation or had a tracheostomy placed. The overall success rate, if all recurrences were considered, was 91%, and 96% if only recalcitrant recurrences were considered.
Advantages
A definitive single-staged surgical resection and reconstruction is the preferred method of management for patients with idiopathic subglottic stenosis and can be performed with excellent short and long term outcomes, high patient satisfaction and rare subsequent progression of the disease. This contrasts with other reports of treatment, largely by laser and dilation, which are often repeatedly required because of recurrence and progression and may ultimately lead to tracheostomy.
Caveats
Avoiding anastomotic tension and devascularization are the keys to minimizing complications in laryngotracheal resection and reconstruction. In general, resections greater than 4 cm should include additional maneuvers to avoid anastomotic tension. Standard techniques include dissection of the pretracheal plane, neck flexion, traction sutures and suprahyoid laryngeal release in extreme cases.
Acknowledgements
The authors wish to acknowledge and thank Edith Tagrin for preparing the initial medical illustrations for this manuscript and Beth Croce for preparing the final illustrations for this publication.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Grillo HC, Mark EJ, Mathisen DJ, et al. Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg 1993;56:80-7. [Crossref] [PubMed]
- Liberman M, Mathisen DJ. Treatment of idiopathic laryngotracheal stenosis. Semin Thorac Cardiovasc Surg 2009;21:278-83. [Crossref] [PubMed]
- Ashiku SK, Kuzucu A, Grillo HC, et al. Idiopathic laryngotracheal stenosis: effective definitive treatment with laryngotracheal resection. J Thorac Cardiovasc Surg 2004;127:99-107. [Crossref] [PubMed]
- Liberman M, Mathisen DJ. Tailored cricoplasty: an improved modification for reconstruction in subglottic tracheal stenosis. J Thorac Cardiovasc Surg 2009;137:573-8; discussion 578-9. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ, Wain JC. Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 1992;53:54-63. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ, Ashiku SK, et al. Successful treatment of idiopathic laryngotracheal stenosis by resection and primary anastomosis. Ann Otol Rhinol Laryngol 2003;112:798-800. [Crossref] [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [Crossref] [PubMed]
- Lanuti M, Mathisen DJ. Management of complications of tracheal surgery. Chest Surg Clin N Am 2003;13:385-97. [Crossref] [PubMed]
- Wang H, Wright CD, Wain JC, et al. Idiopathic Subglottic Stenosis: Factors Affecting Outcome After Single-Stage Repair. Ann Thorac Surg 2015;100:1804-11. [Crossref] [PubMed]