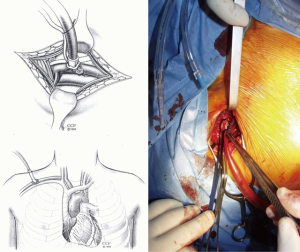
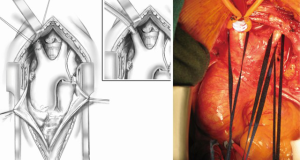
Open repair techniques in the aortic arch are still superior
Introduction
The large variety of methods and techniques used for approaching the aortic arch, protecting the encephalic structures, replacing the diseased aorta, and re-implanting the brachiocephalic vessels, attests the difficulties that the surgeon has to face and accounts for the mortality and morbidity still associated with surgery of the aortic arch.
During the three decades when the so-called ‘conventional techniques’ were developed, their use and practices were not questioned. Since that time, through spectacular breakthroughs or slow evolutions, aortic surgery and aortic arch replacement in replacement seemed to reached their maturity in the nineties. And one can say without a high risk of being wrong that the benefits of techniques such as the use of deep and moderate hypothermia, the use of circulatory arrest during the performance of the anastomoses, the selective cerebral perfusion, the distal aortic perfusion, and CSF drainage, were no longer challenged.
Nevertheless, the rules of aortic repair were totally changed in the early nineties when some surgeons, facing the incredible success of interventional cardiology and radiology, had the idea of treating the aortic diseases with endo-prostheses and stents. Many groups entered the fray and started to treat the aortic lesions using those techniques.
Some reasons were behind this new trend:
- Legitimately preserve patients from very invasive, painful, and risky operations;
- More importantly, the fact that it appeared that some severely ill patients were denied surgery and that some lesions for which surgery was resulting in very poor results, could be efficiently treated.
Going further, some groups thought that those techniques could be substituted to open surgery in aortic segments so far considered as inappropriate for endo-prostheses such as the aortic arch. They therefore invented the so-called “hybrid” or “debranching” procedures. They inferred that in doing so, they would dramatically reduce the mortality and complications of conventional open surgery.
Consequently, hybrid procedures with supra-aortic vessels de-branching methods seem in the present days not only to become more and more popular but also to be considered by some surgeons as a good alternative to the traditional ‘anatomical’ replacement of the arch.
Is it so?
In the present review, we will analyse the “conventional methods” and the “hybrid” ones through the various stages of the procedures and compare the outcomes of both techniques.
The conventional open replacement of the aortic arch
Since the first aortic arch replacements performed during the fifties (1), this specialty has been steadily improving. Considered for a long time as a surgical challenge with major risks and uncertain results, it has become, over 50 years, an essential element of cardiovascular surgery, performed in many centers throughout the world with reproducible and reliable techniques and achievements.
The surgical approach
Classically, the thoracic aorta, from the aortic root to the very proximal descending aorta, may be approached through a sternotomy. This is the easiest, safest and least painful and troublesome approach.
Indeed, it is easy and rapidly performed, it provides an excellent view on the whole anterior aorta (ascending and transverse arch) as well as the origin and the first segments of the supra-aortic vessels, provided, the upper mediastinal fat be dissected free and the innominate vein be divided. It has also the advantage of allowing both pleura to be kept intact and closed and to, consequently, reduce the postoperative pulmonary complications and allow preservation of both internal thoracic arteries for either an immediate or future use if needed. In addition, this approach is, without any doubt, much less painful than any other type of thoracotomy. The drawbacks are few. One is of relatively little importance except in women and young men: the long and important scar. The second drawback is much more deleterious: infection of the site may lead to mediastinitis, the prognosis of which may be quite severe with a mortality reaching 15% to 20% in some recently reported experiences.
To reduce the invasiveness of the sternotomy, it has been proposed during the last decade to limit the opening through partial (mostly upper) sternotomy. Although some few successful experiences have been reported, it seems that for an extended procedure such as total transverse aortic arch replacement such an approach may be questioned, as the opening and the view on the total arch is limited, and as any kind of complication during the surgical procedure would require and immediate conversion to a full sternotomy.
But, whatever its extension, the median sternotomy does not allow easy and safe working on the distal arch beyond the origin of the left subclavian artery and on the descending aorta. In such cases, a left anterior thoracotomy can be associated with the sternotomy in the 5th or 6th intercostal space. But again, this manoeuvre implies a larger rate of postoperative complications.
If a necessary replacement of the whole descending aorta, in addition to the replacement of the aortic arch is required, it was proposed in the late nineties to use the “arch first technique” during which a double thoracotomy with a transverse section of the sternum or so called “clam-shell technique” be used (2). It provides an excellent view of the whole thoracic aorta and the heart. Yet, despite the good results published by its promoters, this technique has not gained much popularity, as it is associated with an important rate of postoperative complications.
So, when considered through daily practice or through reported experiences, it is obvious and rather logical that the approach for conventional aortic arch replacement consists of a median sternotomy in about 85% to 90% of the patients.
The arterial cannulation.
Arterial cannulation for the arterial return of blood during cardiopulmonary bypass (CPB) seems of utmost importance during surgery of the aortic arch.
Indeed, such a procedure requires that, if possible:
- The aorta be perfused antegrade;
- Transverse arch replacement and, in particular, the distal anastomosis be carried out in an open manner under distal total circulatory arrest;
- The brain be perfused selectively and antegrade during this time.
However, since the first experiences of aortic arch replacements under CPB in the early sixties (3), cannulation of the femoral arteries was systematically used as the one and only arterial access. This cannulation technique has indeed some advantages:
- It is easy in the great majority of patients;
- It can be carried out rapidly, in particular in patients in poor hemodynamic condition even in cardiac arrest under resuscitation;
- It can be performed before opening the chest which again represents a large advantage in some instances, such as surgery for acute type A dissection;
- The approach through the groin allows concomitant cannulation of the venous system and possible immediate perfusion through total CPB;
- Two sites are available.
Nevertheless, it has taken about three decades to gain awareness that this technique of cannulation could have several important disadvantages and could be the cause of severe intra or postoperative complication.
The retrograde blood flow in the iliac arteries and/or abdominal and thoracic aorta may indeed, be the cause of thrombo-embolic incidents especially in atheromatous or shaggy vessels, or cause of severe malperfusion and even ruptures in case of acute dissection;
For those major reasons this technique has lost its most prominent hegemony and many groups presently have turned to other modes of cannulation.
In 1995, Sabik et al. proposed the use of the right axillary artery as a surrogate to femoral cannulation (4). This appeared as an important breakthrough in the field and the method gained a certain acceptance rapidly. It indeed concentrates many advantages. In particular, it can be performed before opening the chest, it allows permanent antegrade perfusion of the aorta and selective cerebral perfusion without interruption and prevents from the necessity of switching the arterial cannula to another site when resuming full flow bypass (Figure 1).
Some drawbacks have been described:
- It may be time-consuming, particularly in obese patients;
- The vessel might be somewhat fragile in some instances;
- It is surrounded by several important venous and neural structures;
- In the case of direct and prolonged cannulation, the upper limb might be exposed to some degree of ischemic injury.
So, debate rapidly arose: “Should the right axillary artery be cannulated directly or through a side graft?” It does not seem to make much difference but some papers have reported some accidents and in 2004, Sabik et al. published a comparative study proving that the use of a side graft was significantly favourable in terms of reduction of local disorders (5).
Many groups adopted the technique and several articles reported dramatic improvements in their results (6-9). The technique became, then, the technique of choice for a large number of groups worldwide.
The cannulation of the innominate artery has the same advantages over the axillary artery access. The technique of cannulation is similar to the one used for the aorta (Figure 2). Recently it has been proposed to carry out innominate artery cannulation through a prosthetic Dacron® tube, implanted laterally on the vessel (10). Considering the usual size of the innominate artery, the easiness and the safety of direct cannulation, it seems that this is overly complicated and unnecessary. This technique has been in the recent years reverted to its original form (11). As a matter of fact, it has been used by some groups like ours for several decades and has been regularly part of the usual surgical armamentarium.
In 2006, Urbanski and co-workers proposed to use the common carotid arteries as the arterial access. This technique has many advantages that it shares with the cannulation of the right axillary artery. In addition, it seems to be easier and faster in most patients (12,13).
But it also has certain drawbacks that may prevent most groups from adopting it. The whole perfusion of the brain through this single approach has been questioned, especially when considering that the authors have often used the left carotid regardless of the quality of the circle of Willis. However, their results have been outstanding (14).
The cerebral protection
The repair or replacement of the aortic arch requires a time of arch vessels exclusion. Several techniques of cerebral protection have been described and used in the last five decades.
In a normal human adult, the brain weighs about 1,400 gm, representing 2% to 4% of the body weight. By contrast, the mean cerebral flow represents about 16% of the total cardiac output. These figures stress out the enormous metabolic demand of the brain and the absolute necessity to preserve this metabolic activity.
Normally, the autoregulation of the cerebral flow is maintained for a wide spectrum of mean arterial pressure (50 to 150 mmHg) and there is no relationship between the cerebral blood flow and the level of the mean arterial pressure. However, this autoregulation disappears below 15 °C, at which there is a direct relation between flow and perfusion pressure.
It has been largely demonstrated that an essential determinant of this protection is the use of a certain degree of hypothermia. In 1975, Griepp et al. described the use of profound hypothermia associated to total circulatory arrest during arch replacement (15). Because of its simple use, of the absence of any sophisticated CPB circuit and particular cannulation mode and mostly because it allows the aortic repair to be performed in a totally “blood-less, open” manner, this method was considered as a major progress and became in a few years universally accepted.
Hypothermia markedly reduces oxygen demand and the cerebral metabolism in general. But we should never forget that, contrary to what has been thought for decades, whatever the temperature, this metabolism is never reduced to 0 (16).
But through growing experience, evidence of the limitation of this method came out and criticism emerged from many reports. In particular, the technique implies a long time of CPB to reach a core temperature of 18 °C, and a longer time to re-warm the patient to 36 °C, when the aortic repair is completed. In addition, it has been demonstrated that when the time of cerebral circulation exclusion exceeds 45 minutes, the rate of neurological injuries increases dramatically.
Yet, the method still has its upholders. For instance, recently, the Yale group has published a report with a large cohort of patients (17). Nevertheless, it seems that, presently, the use of profound hypothermia as the only mode of cerebral protection mostly belongs to history and must only be used in exceptional circumstances when nothing else is possible.
Deep hypothermia was also the basic condition for the success of the “retrograde perfusion” method proposed by Ueda et al. (18). It was a good idea rapidly adopted but we know now that, clinically, it could not fulfil its promoters’ expectations.
This was rapidly confirmed by excellent and undisputable experimental studies (19,20).
Because of those drawbacks, our group in Paris described a method of antegrade selective cerebral perfusion associated to moderate core hypothermia, thus suppressing the drawbacks of profound hypothermia yet maintaining the advantages of the circulatory arrest and allowing a less limited time for the aortic repair (21,22).
Our choice of a very low temperature (10 to 12 °C) of the blood perfusate irrigating the brain emerged from the good results of cold cardioplegia. We thought that the same criteria of safety could be applied to the cerebrum. The well-founded rationale of this technique was confirmed by several experimental studies (23,24).
Clinically, we could observe an excellent brain protection as demonstrated by the systematic recording of the EEG during the surgical procedure. EEG silence was obtained in a mean time of 9±6 (range, 3–16) minutes and during re-warming, the first electric wave was seen after 12±15 (range, 1–35) minutes and normal activity restored after a mean of 66±55 (range, 10–210) minutes (25).
A short time later, in Japan, Kazui et al. described a similar method but in which the core and perfusate temperatures are the same resulting in a simplified circuit (26). Because of its greater simplicity, this method has become presently the most popular and used one.
This was confirmed in some experimental (27) and many studies reporting on a fairly large number of patients in whom the mean duration of the distal circulatory arrest was maintained below 45 minutes, even though those experiences included a fair rate of emergency procedures (28,29).
However, a certain number of issues remain for which we have incomplete or no responses or which remain rather controversial.
The necessity for one, two, or even three vessel perfusion represents one of those unresolved controversies. It started with two clinical studies in which only unilateral perfusion from the right axillary or the brachial artery was used (8,30). The results were excellent in terms of postoperative neurological disorders.
Other groups confirmed those results. In a large meta-analysis, Malvindi and co-workers reported the results of several papers in which the authors had used only unilateral SACP. The overall mean rate of neurologic disorders either transient or permanent was 3.8% with a range of 0 to 7% (31).
However, they found 17 papers including 3,548 patients undergoing aortic surgery using either unilateral or bilateral cerebral perfusion. Both methods of cerebral perfusion resulted in low rates of neurological injury. However, the duration of antegrade cerebral perfusion allowed by bilateral perfusion was significantly higher (97 vs. 32 minutes). Therefore, the authors concluded that once the ASCP time is expected to rise over 40 min, bilateral cerebral perfusion is the technique that is best documented to be safe (31).
Urbanski and co-workers have evaluated the role of the anatomical completeness of the circle of Willis. It was normal in only 60% of their patients. However, during unilateral cerebral perfusion, the flow velocity in the contro-lateral middle cerebral artery varied considerably, but the flow never ceased. They concluded that the anatomical status of the circle of Willis does not correlate with functional and intra-operative tests examining the cerebral cross-perfusion (32).
Their experience was in contradiction with other published ones (33,34).
The aortic replacement
Techniques of replacement of the aortic arch are variable and numerous. However, they all start by a complete dissection and control of the transverse arch and the first centimetres of the supra-aortic vessels and ends a few centimetres beyond the origin of the left subclavian artery.
Except in cases of re-do procedures, of very large arch aneurysms or severely jeopardised aorta, this is relatively easy in most patients.
It is made easier by clamping, dividing, and ligating the innominate vein. This manoeuvre gives a perfect view of the whole transverse arch and the first centimetres of its tributaries. It is without any clinical consequences, except in very few patients, some swelling of the left upper limb that resolves in a few days.
If any procedure has to be carried out on the proximal aorta or on other structures of the heart (valvular repair or replacement), it should be done during the time of cooling the patient and distal ascending aorta cross-clamping.
The distal anastomosis
When the proper level of core hypothermia is obtained, distal circulatory arrest is initiated and the transverse arch opened. If a bilateral perfusion mode is chosen, a small balloon cannula is then inserted in the origin of the left common carotid artery and even in the left subclavian artery as proposed by some groups. Otherwise the left subclavian artery must be cross-clamped to avoid blood return and possible steal syndrome of the posterior cerebral structures.
The transverse arch is then resected and the distal anastomosis performed first.
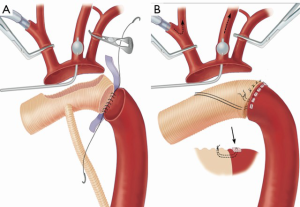
In chronic aneurismal lesions, the aortic wall is generally hardwearing and solid enough to allow tight and solid sutures without the aid of reinforcing artefacts. Nevertheless, some pathologic conditions may require that the anastomosis be reinforced. The commonest and almost only adjunct widely used is Teflon® felt. Some surgeons use it only on demand whereas others use it systematically for all aortic anastomoses. Another trick consists in using U-shaped pledgeted stitches everting the aortic and prosthetic rims (Figure 3A,B).
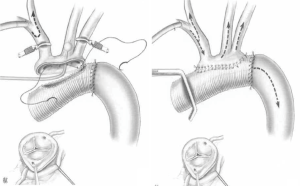
The “Elephant Trunk” technique
In some patients with dilated or jeopardized descending aorta or with connective tissue disease and a significant probability of aneurismal evolution requiring further re-operations, the technique of “Elephant trunk” may be quite useful.
First described by Borst et al. in 1983 (35) and modified by Svensson in 1992 (36) (Figure 4), it consists of folding asymmetrically, a long-enough prosthesis with the longer part inverted into the shorter one. The prosthesis is then placed in the descending aorta and sutured on the trans-section of the descending aorta at the site of the fold. This has the advantage of allowing one single suture and of performing a safer and tighter suture thanks to the double layer prosthetic part. The longer part of the prosthesis is then retrieved and used for the transverse arch replacement.
A large number of immediate and late results of thoracic aortic replacements using this technique have been published. They have generally reported excellent results even when both first and second stage operations were included (37-39).
Yet, during the same period of time, some complications and important issues appeared that somewhat shaded the enthusiasm for the technique.
The most important issues were not technical. They consisted in three main problems:
- When to perform the second stage procedure?
- The reduced but undeniable mortality during the interval between the first and second procedures;
- The rate of morbidity and mortality after the second stage procedure was not negligible and could explain partly the fact that, either for psychological, clinical or other reasons, more than 45% patients never underwent the second stage procedure.
The “Frozen Elephant Trunk” technique
After the development of the graft-stenting of the descending aorta following the first report by Dake et al. (40), the idea that such a technique could be used antegradely during the replacement of the transverse arch came out and rapidly gained interest. This procedure combines the concepts of the elephant trunk principle and endovascular stenting of descending aortic aneurysms. After the very first experiences published in Japan (41), the technique was popularised by the Hannover group (42) and called “the Frozen Elephant technique” by Borst.
Although it associates the use of a stented Dacron graft to the conventional technique of transverse arch replacement, we do not consider this method as a “Hybrid procedure” of aortic arch replacement. The endo-prosthesis has indeed no role in the repair of the transverse aortic arch itself, except for the distal anastomosis. It is placed in the descending aorta and used as a distal adjunct to the conventional replacement of the transverse arch and is placed antegradely during the time of hypothermic circulatory arrest.
During the last two decades several prostheses have been developed industrially and used (Figure 5A,B).
Logically it seems that this technique has not influenced much the immediate results. Conversely the late results seem quite satisfactory. In particular the false lumen in the descending aorta thromboses in a large proportion of patients, reducing the risk for further late distal malperfusion syndromes. Several groups have, indeed, published quite satisfactory results with the use of this method, in particular after surgery for acute or chronic aortic dissection (43-46).
The reimplantation of the supra-aortic vessels
Reimplantation of the three vessels supplying the upper limbs and the brain may be performed either “en bloc” by dividing the area from which they arise on the convexity of the transverse arch and suturing it to an adequately sized opening cut in the prosthesis. The transverse arch should be totally resected, leaving only a small cuff containing the three orifices of the vessels. The cuff is then secured to the prosthesis by means of a 4-0 polypropylene continuous suture (Figure 6).
The reimplantation is increasingly being carried out “separately”, by re-implanting each vessel into the prosthesis. Presently various industrially prepared prostheses are available on the market. The choice of the method depends obviously on the type, location and size of the aneurysm and on the lesions present on the brachiocephalic vessels. But it seems that this choice also depends on the surgical culture and education of the surgeons and has been particularly developed in Japan and Eastern Asia (Figure 7).

The results
During the last three decades, many groups have adopted the techniques described here above or some variations of those. A very large number of articles have reported their experiences and it would be too difficult and long to analyse this important literature. However, we have chosen to indicate here some important articles summarizing well enough what is generally reported.
Those results are indicated on Table 1.
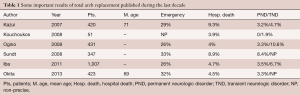
Full table
Kazui and co-workers reported in 2007 their experience of 472 patients operated on between 1986 and 2006 and who had undergone aortic arch replacement with selective cerebral perfusion, moderate core hypothermia and distal circulatory arrest during the time of distal repair (47).
Sundt and colleagues published an interesting study in which they compared the immediate outcomes of patients operated on for aortic arch replacement with three different methods of cerebral protection. Total arch replacement was performed in 95 patients. Isolated deep hypothermic associated circulatory arrest was used in 26 patients, retrograde selective perfusion in deep hypothermia and circulatory arrest in 19 patients and selective antegrade cerebral perfusion in moderate hypothermia and distal circulatory arrest in 50 patients.
The mortality rate for total arch replacement has declined from 34.6% with profound hypothermia and circulatory arrest to 21.1% with retrograde cerebral perfusion and to 6.0% with selective antegrade cerebral perfusion. The corresponding stroke rates were 19.2% with profound hypothermia and circulatory arrest, 5.3% with retrograde cerebral perfusion, and 6.0% with selective antegrade cerebral perfusion (29).
In 2013, Iba and colleagues published their very large experience of conventional total replacement of the arch (48).
The same year, Okita and co-workers published their large experience of 423 consecutive patients who underwent total arch replacement using antegrade selective cerebral perfusion (49).
The hybrid techniques
The rationale
The rationales behind those techniques can be summarized as follows. As a whole they are based on the presumed high-risk status of the patient, on the reduced invasiveness of the procedures, on the easier surgical technique and great technical success and therefore on the better outcomes, considering that, a single-stage approach would be more favorable especially in patients with extensive disease of the aortic arch and descending aorta.
In addition, we may say that the surgical “philosophy” on which those methods are based is quite different than the one supporting conventional arch replacement. As stated by Koullias et al., “this group of hybrid arch repair treats the endovascular repair as the primary arch repair method (meaning the endovascular stent-graft excludes the arch disease without surgically replacing the arch) and the open surgical component is an adjunctive procedure to revascularize the great vessels. Fundamentally, this is quite a different approach to aortic arch disease because it bases the repair not on traditional open surgical techniques, but on the assumption that current endovascular technology can successfully exclude aortic arch diseases and that the arch does not need to be replaced.” (50).
The techniques
In its principle, hybrid repair implies surgical arch “debranching” of the supra-aortic vessels, thereby creating a proximal landing zone of adequate length, and insertion of an endovascular stent graft in the surgically constructed landing zone within the aortic arch. During hybrid repair, the endovascular intervention can be carried out in isolation or concurrently with the surgical intervention.
But the number of vessels, the modes and the place of reimplantation after debranching are quite variable according to the extension of the repair, the lesions, and the surgeons’ preferences.
In order to adequately describe and classify the various techniques and the extension of the endoluminal stenting, Criado and colleagues defined the proximal landing zone as: zone 0 (proximal to the innominate artery), zone 1 (between the innominate and left common carotid artery), zone 2 (between the left common carotid and subclavian arteries), and zone 3 (distal of the left subclavian artery) (51).
Some procedures requiring landing zone 2 or 3 may be carried out after the completion of extra-anatomic bypasses to the head vessels with subsequent endoluminal stent-grafting, without sternotomy and open debranching (52).
Many techniques have been used such as the common right to left carotid-carotid bypass, right subclavian to left subclavian bypass or any combinations of those techniques (Figure 8).
Those various modes of bypasses are too numerous to be described here in details.
The most frequent extra-anatomic bypass during the hybrid procedure is, however, performed between the left subclavian and the left common carotid artery either directly or with the aid of a short graft. The benefit of a left carotid subclavian bypass procedure is that only two cerebral vessels (the innominate and left common carotid artery) need to be addressed at the time of the aortic arch repair, decreasing the duration of cerebral ischemia.
This bypass can be performed through a left supra-clavicular approach or through the upper part of the sternotomy with a small left extension of the incision to the left.
In the present review we will focus our description on the procedures requiring a zone 0 landing zone, in which the entire aortic arch requires reconstruction, since they are the only ones that can be appropriately compared to conventional open total replacement of the transverse aortic arch.
In 2003, Czerny and co-workers published what seemed to be the first case of complete sequential transposition of the cerebral vessels before endovascular stent grafting of the aortic arch (53).
Through total median sternotomy, the patient was treated by sequential transposition of the left carotid artery into the brachiocephalic trunk and the left subclavian artery into the previously transposed left common carotid artery, with subsequent endovascular stent-graft placement into the aortic arch (zone 1 landing zone) one day later.
In 2010, Bavaria and colleagues published their experience initiated in 2005 with the technique of debranching and re-implanting the supra-aortic vessels through a trifurcated graft. They defined two technical types: type I when the graft can be directly implanted on the proximal part of the native ascending aorta; type II when the ascending aorta has to be replaced and the trifurcated graft anastomosed to the main aortic graft. In both types the stent-graft is placed antegradely in the arch immediately after the completion of the debranching procedure through an additional branch (54).
Going further, Esposito and co-workers described a prosthesis called “Lupiae prosthesis” in which the trifurcated graft allowing reimplantation of the debranched supra-aortic vessels is industrially implanted on a regular aortic graft used for replacement of the ascending aorta and hemi or total arch and providing a good landing zone for immediate or further endoluminal stent-grafting of the distal aorta.
In 2012 they published their experience with this technique in patients suffering from mega-aorta syndrome and in 2015 their 10-year experience in patients operated on for acute type A dissections (55,56) (Figure 9).
Many variations of those main techniques of “debranching” and “hybrid procedures” have been described and can be found in the literature. Most of those variations, however, stem from the main rationale and use more or less similar technical processes as the ones described here above.
Yet, as for the conventional open replacement of the aortic arch., several issues remain unresolved or poorly defined. This may shed some uncertainty over the published results and make the comparison with other modes of treatment, and in particular with conventional open replacement of the total aortic arch, somewhat difficult and in some instances, irrelevant.
The reduced mortality and morbidity
Despite the fact that the hybrid techniques for aortic arch repair have become popular and performed in many centers worldwide, no randomized control trial has been performed yet comparing those methods with the conventional open surgical techniques of total aortic arch replacement. Therefore, the comparison can rely only on the results of reported experiences of single centers, or on meta-analyses.
In 2004, Czerny and colleagues published their initial experience of five patients operated on with the technique of debranching and transposition of the three supra-aortic vessels (57).
In 2011, Geisbüch and colleagues published their experience of 47 patients who underwent a hybrid debranching procedure between 1997 and 2009 (58).
In 2012, Czerny and co-workers, published the experience of five European and North-American centers (59).
In a recent large case series, Bavaria and colleagues reported on the results of 47 patients who underwent extensive hybrid arch repairs with either antegrade or retrograde stent-grafting of the aortic arch (60).
In 2013, De Rango et al. published a series of 104 consecutive patients with elective debranching and TEVAR including 19 patients with zone 0 disease requiring total debranching (61).
In the same year, Andersen and colleagues published their experience. Between August 2005 and January 2012, among a cohort of 87 patients undergoing hybrid procedures of various types, 48 patients underwent hybrid arch repair with zone 0 endograft landing zone and aortic arch debranching (62).
The results of the here above indicated studies are shown in Table 2.
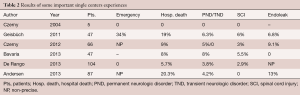
Full table
Very few meta-analyses were published but they overall confirmed the results of the large single centers experiences published during the same period of time. This is the case for instance, of the meta-analysis published in 2010 by Koullias et al. that included 463 patients who underwent hybrid arch surgery (50).
In 2012, Cao and co-workers published a large meta-analysis of the literature concerning the results of the hybrid procedures for treatment of the aortic arch diseases. Studies involving hybrid aortic arch procedures [2002–2011] were systematically searched and reviewed. End points were peri-procedural mortality, stroke, and spinal cord ischemia (63) (see Table 3).

Full table
Obviously, those results have to be compared with the results obtained in open replacement of the total arch through the use of conventional techniques as described in the first part of this chapter.
In recent years, several large meta-analyses comparing open surgery and hybrid methods to benchmark this innovative approach were conducted in order to better assess technical success, stroke, spinal cord ischemia (SCI), renal failure and cardiac and pulmonary complications rate, as well as in-hospital mortality, and try to determinate whether or not the hybrid techniques were representing a clear improvement in the treatment of aortic arch diseases.
A few reports comparing both conventional total arch replacement and hybrid debranching methods with total arch endo-grafting to zone 0, either performed by the same groups or reported through meta-analyses, are available in the literature. The most important ones are summarized in Table 4.
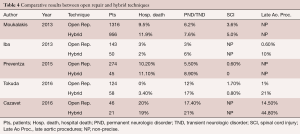
Full table
In a recent study, Moulakakis and co-workers have identified all articles published up to December 2012 that described hybrid aortic arch repair with intra-thoracic supra-aortic branch revascularization and subsequent stent graft deployment and compared this group with the patients who underwent conventional arch replacement with an elephant trunk technique (64).
In 2013, Iba and colleagues published a comparative study of 143 patients who had undergone a conventional arch replacement with 50 patients who had undergone a hybrid procedure, between 2008 and 2013 (65).
Preventza and co-workers published in 2015 a comparative study of zone 0 hybrid arch exclusion versus traditional open repair (66).
In 2016, Tokuda and co-workers published a comparative study of the patients who underwent either hybrid debranching or conventional open aortic arch replacement in their institution between 2002 and 2014 (67).
Similarly, Cazavet and colleagues recently published their comparative experience of 46 patients operated on for exclusive aortic arch aneurysm involving zone 0 or zone 1 (68).
Discussion
When analyzing the various reports concerning the techniques of hybrid debranching arch repair recently published and comparing them to the reported experiences of conventional open total replacement of the aortic arch, we may observe that, in those latter reports, the number of patients is much more important and that the proportion of emergencies was much higher.
It is also noteworthy that in most reports concerning the hybrid methods or in the available meta-analyses, the mortality and neurological complications rates are not obviously lower than the ones reported with conventional surgery and that, in some instances they seem largely superior and the overall outcomes obviously not better than in conventional arch replacement.
The promoters and upholders of the hybrid techniques repeatedly argue in their publications that the advantages of the methods are:
- Their possible use in risky patients not or poorly amenable to conventional open surgery;
- Their reduced invasiveness;
- The easier surgical techniques;
- The immediate technical success.
Those points are indeed rather appealing and no one could question their advantage. The problem is that it appears through the reported experiences and the observed daily practice that those factors of improvement are not systematically present or even often largely flouted.
The risky patient
How are the patients’ risks assessed and what is a ‘risky patient’? This major question is very seldom addressed. In most articles, the EuroSCORE or the STS score or any other score do not appear. By the way, it is of note that, in most articles dealing with this matter, when one of these scores is calculated, it is often not worse than for patients undergoing conventional surgery, and the threshold beyond which the patients are considered ‘non-amenable to open surgery’ is never objectively defined. So, should we infer that the risk estimation is mostly left to the surgeons?
In addition and somewhat paradoxically, it is to be noticed that in many reports or in daily practice, when a patient who underwent any hybrid procedure because of his/her “risky condition”, suffers from a severe immediate or delayed complication, he generally is re-operated on in an open conventional manner. In this regard one may observe that the hybrid procedures are responsible for three severe and immediate or delayed complications, that are almost completely ignored with the conventional open methods (except in case of Frozen Elephant Trunk): retrograde acute dissection, spinal cord injury and endoleaks.
The reduced invasiveness
Hybrid procedures are generally considered as minimally invasive. It is interesting to observe that many patients who are supposed to be in too poor a condition to undergo conventional surgery have to undergo a median sternotomy, and, in many cases, two to four peripheral vascular approaches.
In a meta-analysis, Koullias et al., have shown that such vascular approaches are present in more than 60% of the patients having any kind of debranching procedure and that, obviously opening of the chest is carried out in all patients in whom zone 0 is concerned (50). In addition, one may observe that in a certain proportion of patients, one or even several additional vascular sites (supra-clavicular or groin approach) are necessary to perform either the supra-aortic vessels reimplantation or the insertion of the stent-graft.
Is such a procedure really less invasive and more appropriate than a straightforward conventional replacement of the aortic arch?
The easier surgical technique
Hybrid debranching procedures have the reputation of being surgically easier. This is obviously true if we consider the patients requiring only one or two subclavian carotid or carotid-carotid bypasses. But as soon as we deal with real complex aortic arch lesions it is somewhat difficult to observe any simpler surgery than the conventional one. Indeed, the thorax has to be opened, the supra-aortic vessels dissected free and controlled. If the ascending aorta or the aortic valve or both are not to be replaced, a side bite cross clamping of the ascending aorta (possible factor of stroke in cases of local atheroma) has to be carried out. Then, an end-to-side anastomosis of a bi or trifurcated graft has to be performed. If the ascending aorta or the aortic valve or both are to be replaced, then CPB is necessary, sometimes associated with hypothermia and eventually some circulatory arrest before re-implanting sequentially all the supra-aortic vessels. Lastly, the anastomosis on the distal aortic stump has no reason to be simpler than in conventional surgery, especially if the hybrid procedure is carried out for some acute dissection.
This is quite obvious when we analyze methods such as the Lupiae technique, for instance, that appear as rather complex.
And if we look at the mean number of required anastomoses in some reported cases, as well as the number and length of the various Dacron conduits used to re-implant the supra-aortic vessels, one may wonder how such methods are “simpler” than conventional aortic arch replacement even associated to the Frozen Elephant Trunk technique (Figure 10) (64).
The technical success
The notion of immediate ‘technical’ success, which has been developed by the interventional community, represents a weird feature. What is its meaning if it is isolated from the only valid and acceptable ‘success’ that is the clinical and physiological one? Furthermore, what is the meaning of a ‘clinical success’ at 30 days postoperatively? Traditionally and statistically, surgical techniques are assessed through the hospital, mid- and long-term mortality and morbidity rates (in general 1, 5 and even 10 years of follow-up). Only such data can allow stating that a technique is legitimate and may constitute a therapeutic method of choice or, at least, an alternative to a previously validated method.
Additionally, in most publications reporting the results of hybrid procedures, the mean follow-up is rather short and does not allow having a proper and accurate idea of the long-term outcome of those techniques. It is indeed noteworthy that in most reported experiences as well as in the meta-analyses, the rates of late aortic incidents, late re-interventions or surgical procedures are significantly higher than with the conventional replacement methods.
Therefore, it seems that no obvious evidence supports the superiority of the hybrid techniques relative to open arch replacement.
Contrary to what could be expected, the hybrid repair of the aortic arch carries not negligible risks of perioperative mortality and neurological morbidity, and is associated to higher rates of late complications.
Yet, we agree with Iba and co-workers stating:
“Hybrid arch TEVAR is still in a developing stage and new techniques such as the chimney stent graft technique, or new fenestrated or branched devices are under trial; however, we now advocate that the extended application of this new technology to patients with a reasonable risk should be reconsidered according to the results of the present studies. In any case, we should reconsider the classification criteria for regarding patients as high risk for aortic repair. Furthermore, we suggest that the establishment of a ‘risk-oriented strategy’ based on a proper risk evaluation for aortic repair is an important issue to be addressed in the future. In conclusion, the recent outcomes of open arch repair and hybrid TEVAR demonstrate acceptable results, particularly early after the procedure; however, open arch repair provides more reliable outcomes in follow-up.” (65).
Conclusions
So, conventional arch replacement can be carried out in a great majority of patients. Hybrid procedures are often as invasive and technically difficult as conventional ones. Moreover, their immediate results are, in many reported experiences, not better and their long-term results less favourable than the ones observed with conventional methods. So, yes, the open conventional arch replacement is still “the gold standard”.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- De Bakey ME, Crawford ES, Cooley DA, et al. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg Gynecol Obstet 1957;105:657-64. [PubMed]
- Rokkas CK, Kouchoukos NT. Single -stage extensive replacement of the thoracic aorta: the arch-first technique. J Thorac Cardiovasc Surg 1999;117:99-105. [Crossref] [PubMed]
- De Bakey ME, Beall AC, Cooley DA, et al. Resection and graft replacement of aneurysms involving the transverse arch of the aorta. Surg Clin North Am 1966;46:1057-71. [Crossref] [PubMed]
- Sabik JF, Lytle BW, McCarthy PM, et al. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg 1995;109:885-90. [Crossref] [PubMed]
- Sabik JF, Nemeh H, Lytle BW, et al. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg 2004;77:1315-20. [Crossref] [PubMed]
- Pasic M, Schubel J, Bauer M, et al. Cannulation of the right axillary artery for surgery of acute type A aortic dissection. Eur J Cardiothorac Surg 2003;24:231-5; discussion 235-6. [Crossref] [PubMed]
- Moizumi Y, Motoyoshi N, Sakuma K, et al. Axillary artery cannulation improves operative results for acute type A aortic dissection. Ann Thorac Surg 2005;80:77-83. [Crossref] [PubMed]
- Reuthebuch O, Schurr U, Hellermann J, et al. Advantages of subclavian artery perfusion for repair of acute type A dissection. Eur J Cardiothorac Surg 2004;26:592-8. [Crossref] [PubMed]
- Etz CD, Plestis KA, Kari FA, et al. Axillary cannulation significantly improves survival and neurologic outcome after atherosclerotic aneurysm repair of the aortic root and ascending aorta. Ann Thorac Surg 2008;86:441-6; discussion 446-7. [Crossref] [PubMed]
- Di Eusanio M, Ciano M, Labriola G, et al. Cannulation of the innominate artery during surgery of the thoracic aorta: our experience in 55 patients. Eur J Cardiothorac Surg 2007;32:270-3. [Crossref] [PubMed]
- Preventza O, Bakaeen FG, Stephens EH, et al. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg 2013;145:S191-6. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Lindemann Y, et al. Carotid artery cannulation in aortic surgery. J Thorac Cardiovasc Surg 2006;132:1398-403. [Crossref] [PubMed]
- Urbanski PP, Sabik JF, Bachet JE. Cannulation of an arch artery for hostile aorta. Eur J Cardiothorac Surg 2017;51:2-9. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Blume JC, et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur J Cardiothorac Surg 2008;33:402-8. [Crossref] [PubMed]
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Ehrlich MP, McCullough JN, Zhang N, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73:191-7. [Crossref] [PubMed]
- Gega A, Rizzo JA, Johnson MH, et al. Straight Deep Hypothermic Arrest: Experience in 394 Patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84:759-66. [Crossref] [PubMed]
- Ueda Y, Miki S, Kusuhara K, et al. Deep hypothermic systemic circulatory arrest and continuous retrograde cerebral perfusion for surgery of aortic arch aneurysm. Eur J Cardiothorac Surg 1992;6:36-41; discussion 42. [Crossref] [PubMed]
- Boeckxstaens CJ, Flameng WJ. Retrograde cerebral perfusion does not perfuse the brain in nonhuman primates. Ann Thorac Surg 1995;60:319-27; discussion 327-8. [Crossref]
- de Brux JL, Subayi JB, Pegis JD, et al. Retrograde Cerebral Perfusion: Anatomic Study of the Distribution of Blood to the Brain. Ann Thorac Surg 1995;60:1294-8. [Crossref] [PubMed]
- Guilmet D, Roux PM, Bachet J, et al. Nouvelle technique de protection cérébrale dans la chirurgie de la crosse aortique. N Presse Med 1986;15:1096-8.
- Bachet J, Guilmet D, Goudot B, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102:85-93. [PubMed]
- Swain JA, McDonald TJ Jr, Griffin PK, et al. Low-flow hypothermic Cardio-Pulmonary Bypass protects the brain. J Thorac Cardiovasc Surg 1991;102:76-83; discussion 83-4. [PubMed]
- Filgueiras CL, Winsborrow B, Ye J, et al. A 31p-magnetic resonance study of antegrade and retrograde cerebral perfusion during aortic arch surgery in pigs. J Thorac Cardiovasc Surg 1995;110:55-62. [Crossref] [PubMed]
- Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral protection with cold blood: a 13-year experience. Ann Thorac Surg 1999;67:1874-8; discussion 1891-4.
- Kazui T, Inoue N, Yamada O, et al. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53:109-14. [Crossref] [PubMed]
- Salazar J, Coleman R, Griffith S, et al. Brain preservation with selective cerebral perfusion for operations requiring circulatory arrest: protection at 25 degrees C is similar to 18 degrees C with shorter operating times. Eur J Cardiothorac Surg 2009;36:524-31. [Crossref] [PubMed]
- Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2007;31:618-22. [Crossref] [PubMed]
- Sundt TM, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg 2008;86:787-96. [Crossref] [PubMed]
- Küçüker SA, Ozatik MA, Saritaş A, et al. Arch repair with unilateral antegrade cerebral perfusion. Eur J Cardiothorac Surg 2005;27:638-43. [Crossref] [PubMed]
- Malvindi PG, Scrascia G, Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg 2008;7:891-97. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Blume JC, et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur J Cardiothorac Surg 2008;33:402-8. [Crossref] [PubMed]
- Olsson C, Thelin S. Antegrade cerebral perfusion with a simplified technique: unilateral versus bilateral perfusion. Ann Thorac Surg 2006;81:868-74. [Crossref] [PubMed]
- Krähenbühl ES, Clément M, Reineke M, et al. Antegrade cerebral protection in thoracic aortic surgery: lessons from the past decade. Eur J Cardiothorac Surg 2010;38:46-51. [Crossref] [PubMed]
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Svensson LG. Rationale and technique for replacement of the ascending aorta, arch, and distal aorta using a modified elephant trunk procedure. J Card Surg 1992;7:301-12. [Crossref] [PubMed]
- Schepens MA, Dossche KM, Morshuis WJ, et al. The elephant trunk technique: operative results in 100 consecutive patients. Eur J Cardiothorac Surg 2002;21:276-81. [Crossref] [PubMed]
- Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: long-term experience with the elephant trunk technique. Ann Surg 2004;240:677-84. [PubMed]
- Shrestha M, Martens A, Kruger H, et al. Total aortic arch replacement with the elephant trunk technique: single-centre 30-year results. Eur J Cardiothorac Surg 2014;45:289-95; discussion 295-6. [Crossref] [PubMed]
- Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729-34. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Di Bartolomeo R, Pacini D, Savini C, et al. Complex thoracic aortic disease: single-stage procedure with the frozen elephant trunk technique. J Thorac Cardiovasc Surg 2010;140:S81-5. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. [Crossref] [PubMed]
- Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J Cardiothorac Surg 2017;52:858-66. [Crossref] [PubMed]
- Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg 2007;83:S796-8. [Crossref] [PubMed]
- Iba Y, Minatoya K, Matsuda H, et al. Contemporary open aortic arch repair with selective cerebral perfusion in the era of endovascular aortic repair. J Thorac Cardiovasc Surg 2013;145:S72-77. [Crossref] [PubMed]
- Okita Y, Okada K, Omura A, et al. Total arch replacement using antegrade cerebral perfusion. J Thorac Cardiovasc Surg 2013;145:S63-71. [Crossref] [PubMed]
- Koullias GJ, Wheatley GH 3rd. State-of-the-art of hybrid procedures for the aortic arch: a meta-analysis. Ann Thorac Surg 2010;90:689-97. [Crossref] [PubMed]
- Criado FJ, Barnatan MF, Rizk Y, et al. Technical strategies to expand stent-graft applicability in the aortic arch and proximal descending thoracic aorta. J Endovasc Ther 2002;9:II32-8. [Crossref] [PubMed]
- Zhou W, Reardon M, Peden EK, et al. Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J Vasc Surg 2006;44:688-93. [Crossref] [PubMed]
- Czerny M, Fleck T, Zimpfer D, et al. Combined repair of an aortic arch aneurysm by sequential transposition of the supraaortic branches and consecutive endovascular stent-graft placement. J Thorac Cardiovasc Surg 2003;126:916-8. [Crossref] [PubMed]
- Bavaria J, Milewski RK, Baker J, et al. Classic hybrid evolving approach to distal arch aneurysms: toward the zone zero solution. J Thorac Cardiovasc Surg 2010;140:S77-80. [Crossref] [PubMed]
- Esposito G, Cappabianca G, Ciano M, et al. Mid-term results of the Lupiae technique in patients with De Bakey Type I acute aortic dissection. Eur J Cardiothorac Surg 2012;42:242-7; discussion 247-8. [Crossref] [PubMed]
- Esposito G, Cappabianca G, Bichi S, et al. Hybrid repair of type A acute aortic dissections with the Lupiae technique: ten-year results. J Thorac Cardiovasc Surg 2015;149:S99-104. [Crossref] [PubMed]
- Czerny M, Zimpfer D, Fleck T, et al. Initial results after combined repair of aortic arch aneurysms by sequential transposition of the supra-aortic branches and consecutive endovascular stent-graft placement. Ann Thorac Surg 2004;78:1256-60. [Crossref] [PubMed]
- Geisbüsch P, Kotelis D, Müller-Eschner M, et al. Complications after aortic arch hybrid repair. J Vasc Surg 2011;53:935-41. [Crossref] [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [Crossref] [PubMed]
- Bavaria J, Vallabhajosyula P, Moeller P, et al. Hybrid approaches in the treatment of aortic arch aneurysms: Postoperative and midterm outcomes. J Thorac Cardiovasc Surg 2013;145:S85-90. [Crossref] [PubMed]
- De Rango P, Cao P, Ferrer C, et al. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg 2014;59:107-14. [Crossref] [PubMed]
- Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67. [Crossref] [PubMed]
- Cao P, Rango PD, Czerny M, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. J Thorac Cardiovasc Surg 2012;144:1286-300. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Iba Y, Minatoya K, Matsuda H, et al. How should aortic arch aneurysms be treated in the endovascular aortic repair era? A risk-adjusted comparison between open and hybrid arch repair using propensity score-matching analysis. Eur J Cardiothorac Surg 2014;46:32-9. [Crossref] [PubMed]
- Preventza O, Garcia A, Cooley DA, et al. Total aortic arch replacement: A comparative study of zone 0 hybrid arch exclusion versus traditional open repair. J Thorac Cardiovasc Surg 2015;150:1591-8. [Crossref] [PubMed]
- Tokuda Y, Oshima H, Narita Y, et al. Hybrid versus open repair of aortic arch aneurysms: comparison of postoperative and mid-term outcomes with a propensity score-matching analysis. Eur J Cardiothorac Surg 2016;49:149-56. [Crossref] [PubMed]
- Cazavet A, Alacoque X, Marcheix B, et al. Aortic arch aneurysm: short- and mid-term results comparing open arch surgery and the hybrid procedure. Eur J Cardiothorac Surg 2016;49:134-40. [Crossref] [PubMed]