The risk of mediastinitis and deep sternal wound infections with single and bilateral, pedicled and skeletonized internal thoracic arteries
Introduction
The development of guidelines to prevent sternal wound infections has helped to reduce the incidence of deep sternal wound infections (DSWI) to less than 1% in coronary artery bypass graft (CABG) patients (1,2). Nevertheless, DSWI are associated with increased morbidity and mortality, decreased long-term life expectancy, prolonged hospital length of stay and increased medical costs. DSWIs are now publicly reported and third-party payers in the United States (US) may no longer reimburse hospital costs for DSWI following CABG.
There is now evidence to suggest that the use of bilateral internal thoracic artery (BITA) grafting in CABG patients may prolong survival and decrease the incidence of recurrent ischemia and the need for re-revascularization procedures (3). However, surgeons continue to avoid using BITA grafting because of a perceived increased risk of sternal wound infections, especially in those who are obese, are poorly controlled diabetic patients, or are patients with chronic obstructive pulmonary disease (COPD). In 2016, BITA grafting was used in only 5.5% of CABG procedures in the US, and in 12–15% of CABG patients in Europe (4).
This article will examine the association of BITA grafting with DSWI in CABG patients, discuss the potential role of skeletonization of ITA grafts to reduce DSWI, and review the various interventions that have contributed to the significant decrease of DSWI in CABG surgery.
Methods
Medline and PubMed searches were performed to review the literature from 1970–2017 regarding the effect of ITA grafting on sternal blood flow, the risk of single ITA (SITA) vs. BITA grafting in diabetic and non-diabetic CABG patients, and the techniques and effects of skeletonization vs. pedicled BITAs on DSWIs.
Results
Effects of ITA harvesting on sternal blood flow
The major source of sternal blood flow is from the anterior intercostal arteries, which originate directly or as a common trunk from the ITA (5). Berdajs et al. studied the blood supply to the sternum in 50 human cadavers and found predominately 4 anatomical patterns of collateral vessels, which varied widely amongst individual patients (5). The majority of ITA branches originated at the level of the first three intercostal spaces. There were fewer collateral vessels in the inferior portion of the sternum, making this area more vulnerable to infection following ITA harvest. These anatomical variations highlight the importance of clipping the vessels as close as possible to the ITA, in an effort to avoid injury to the distal portion of the intercostal vessel and preserve collateral blood flow. When the anterior intercostal trunk is left intact after harvesting the ITA, it provides a significant blood flow to the sternum, irrespective of whether the ITA is harvested as a pedicled or skeletonized graft (6). The question then arises as to which of the harvesting techniques best preserves sternal blood flow. Unfortunately, the results have been mixed.
Cohen et al., (using technetium-99 methylene diphosphate bone scanning and single photon-emission CT scanning 4–9 days after surgery, showed that a pedicled left ITA (LITA) reduced sternal blood flow to a greater degree than a skeletonized LITA (7). Parish et al., using radioactive microspheres in dogs, found that skeletonized ITAs resulted in significantly greater sternal blood flow than pedicled grafts (8).
However, other investigators have found no difference in sternal blood flow between pedicled and skeletonized grafts. Kamiya et al. studied changes in sternal blood flow following ITA grafting in 24 non-diabetic males (9). They assessed blood flow using a laser Doppler flow meter and remission spectroscopy to measure both tissue oxygenation and blood flow. They found that presternal and retrosternal blood flow decreased in both skeletonized and pedicled ITAs. Skeletonized ITAs showed no advantage in preserving presternal blood flow, although retrosternal blood flow appeared to be greater in the middle and lower sternum using the skeletonized technique. None of the 24 patients developed a DSWI.
Carrier et al. used sternal bone tomography with technetium 99-methylene diphosphate to study sternal blood flow after SITA, BITA, and saphenous vein graft (SVG) harvesting in both diabetic and non-diabetic patients (10). These studies were performed 7 days and 1 month following surgery. Focal zones of hypoactivity of technetium represented areas of sternal hypoperfusion which were compared as a ratio to the entire sternum. None of the grafts were skeletonized. After 7 days, patients receiving only SVGs had the lowest areas of hypoactivity, and BITA grafting had the highest (4% SVG vs. 14% SITA vs. 24% BITA; P<0.001). However, one month following surgery, areas of hypoperfusion decreased significantly in all the groups such that there was no difference amongst the conduits (2% SVG, 2.2% SITA vs. 2.0% BITA; P>0.05). There was no difference in areas of hypoperfusion between diabetic and non-diabetic patients amongst the various groups. The authors concluded that while pedicled BITA grafting resulted in a significant decrease in sternal blood flow compared to SITA grafting shortly after surgery, this was transient and reverted back to baseline at 1 month. This suggests that collaterals from intramedullary and periosteal blood flow, as well as from the anterior intercostal chain are present to promote sternal healing following ITA harvesting. This implies that factors, other than reduced blood flow, may be responsible for the development of sternal infections following ITA harvesting, irrespective of which harvesting technique is used. One of these factors is the manner in which the sternum is closed. As noted by the recent the American Association for Thoracic Surgery (AATS) guidelines, closing the sternum with a figure-of-8 technique is preferable in the prevention of sternal dehiscence and wound infections. Furthermore, in order to avoid damage to collateral vessels (including perforating branches and superficial and deep vascular arcades) after the ITA have been harvested, sternal wires should be placed as close as possible to the sternal edge to avoid damaging these important sternal collateral vessels.
SITA vs. BITA and the risk of DSWI in diabetic and non-diabetic patients
In 1990, Kouchoukos et al. first reported the relationship between BITA grafting and DSWI (11). Since then, other investigators have reported a higher incidence of DSWI in patients undergoing BITA grafting (12-14). Dai et al., in a meta-analysis of observational studies, found that the use of BITA grafts increased the risk of any sternal infection, deep or superficial, by 62% (15). In the Arterial Revascularization Trial (16), BITA grafting resulted in a higher rate of sternal wound infections (3.5% vs. 1.9%; P=0.005) and need for sternal reconstruction procedures (1.9% vs. 0.6%; P=0.002). However, despite the perception that BITA grafting represents a major risk factor for sternal wound complications, the evidence remains controversial and conflicting results have been reported, as noted in Box 1.
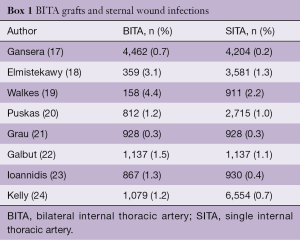
Full table
The explanation for these discrepancies is multifactorial. These studies are observational and retrospective, from different eras, include both diabetic and non-diabetic patients and the method of sternal harvesting is not always reported. Furthermore, there is no mention of glycemic control, antibiotic prophylaxis and the techniques used to close the sternum. The evidence for the relationship between obesity and DSWI and BITA grafting is also inconclusive. In a report of 1,526,360 patients from the US Nationwide Inpatient Sample who underwent isolated CABG, BITA grafting was associated with an increased risk of DSWI only in patients with severe, chronic diabetes, but not obese patients (25). Several other series have also failed to find a relationship between BITA grafting and DSWI in obese patients, as noted in Box 2.
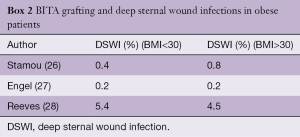
Full table
As previously noted, these are retrospective, observational studies, and the method of sternal harvesting was not always reported. Hirotani et al. reported 420 patients, both diabetic and non-diabetic, undergoing isolated CABG in which tight glycemic control was practiced to achieve a blood glucose <140 mg/dL (29). The method of ITA harvest was not reported. The overall incidence of DSWI was 1.9% in both diabetic and non-diabetic patients. The incidence of DSWI in diabetic patients receiving BITA vs. SITA grafting was 2.7% vs. 1.2% (P>0.05), and in non-diabetic patients, it was 0% vs. 2.3% (P>0.05). LaPar et al. reported on the incidence of DSWI in 43,823 patients undergoing isolated CABG form the Virginia Cardiac Surgery Quality Initiative Data Registry (30). The study was retrospective, and there was no information regarding glycemic control or the method of ITA harvest. BITA grafting was utilized in only 3% of the patients. After propensity matching, there were 1,333 BITA and 1,333 SITA patients, with no significant difference in the incidence of DSWI between the two techniques (0.4% BITA vs. 0.2% SITA; P=0.48).
Skeletonization of the ITA—effects on length, chest wall pain and patency
In view of the concerns of decreased sternal blood flow and increased DSWI with pedicled ITAs, Keeley introduced the skeletonization technique for harvesting ITA grafts in 1987 (31). Skeletonization increased the length of the ITA by as much as 2.5 cm, which could allow it to reach more distal targets (32). Takami et al. found that intraoperative flow was increased in skeletonized ITA grafts, which may prevent the hypoperfusion syndrome that has been observed when smaller pedicled ITA grafts have been used to revascularize large areas of myocardium, especially when they are used as sequential grafts in patients with left ventricular hypertrophy (33).
Pedicled ITA grafts have been associated with increased and persistent chest wall pain (34,35). In a randomized, double-blind study in which 1 ITA was harvested as a pedicle graft and the other was skeletonized, skeletonization resulted in decreased pain and sensory deficits 3 months following surgery (36). The skeletonization technique may result in less damage to the anterior branches of the intercostal nerves, which contribute to the frequency and severity of postoperative chest wall pain. In addition, ultrasonic cauterization, which is frequently used in the skeletonization technique, may decrease damage to the chest wall, as opposed to conventional electrocautery, which is more frequently used in pedicle ITAs. However, in another prospective, randomized double-blind study, Markham et al. found that although chest wall dysesthesia was reduced 7 weeks following surgery in patients with skeletonized ITAs, there was no difference in dysesthesia between pedicled and skeletonized ITAs after 21 weeks (37). Furthermore, there was no difference in chest wall pain between the two techniques at either 7 or 21 weeks following surgery. They concluded that skeletonization provided only a short-term reduction in chest wall dysesthesia following CABG. Chest wall dysesthesia following ITA harvesting is due to injury of the intercostal nerves and subsides as the tissue heals. Another contributing factor to postoperative chest wall discomfort, irrespective of the ITA harvesting technique, is trauma due to chest wall retraction. Therefore, chest wall discomfort following ITA harvesting is multifactorial and may not be solely due to the harvesting technique.
Concerns were initially raised that skeletonization may result in damage to the ITA and alter its protective, anti-atherosclerotic and vasodilatory properties, thus affecting graft patency. However, several studies have demonstrated that skeletonization preserves both the structural and functional integrity of the endothelium, compared to pedicled ITA grafts (38,39). Calafiore et al. found no difference in patency rates between skeletonized and pedicled ITAs in angiographic studies obtained 9 months following surgery (96.8% vs. 94.3%) (40). However, follow-up angiography was only obtained in a third of the patients undergoing ITA grafting. Nevertheless, other studies have found no difference in late clinical outcomes in patients receiving bilateral skeletonized ITAs compared to patients with bilateral pedicle grafts (41).
Skeletonized ITAs and the incidence of DSWI
Several studies examined the effect of skeletonized ITAs on the incidence of DSWI. In all of these studies, only skeletonized ITAs were used during CABG surgery. Sofer et al. reported 545 CABG patients, all of whom had BITA grafts (42). Thirty percent of these patients were diabetics. The incidence of DSWI was 1.7%, and 2.8% had secondary sternal wound infection (SSWI). Diabetes was not a predictor for sternal wound complications. No mention was made of any glycemic control or protocols for perioperative antibiotics. Endo et al. studied the effect of skeletonized SITA and BITA grafting in diabetic and non-diabetic patients with established perioperative glycemic control protocols (43). There was no difference in the incidence of DSWI between diabetic vs. non-diabetic patients (0.9% vs. 0.3%; P=0.24). In non-diabetic patients, there was no difference in the incidence of DSWI amongst SITA vs. BITA grafting (0.2% vs. 0.4%; P=0.99). Similarly, in diabetic patients, there was also no difference in the incidence of DSWI between SITA vs. BITA grafts (1.1% vs. 0.5%; P=0.65). Hemo et al. studied the long-term outcomes in patients with diabetes receiving BITA grafts in 801 patients (44). The overall incidence of any sternal infection was 3.7%. No mention was made of any glycemic control. Sternal wound infections were more common in females, obese patients, and patients with COPD.
From 2000–2006, the use of BITA grafting was more selective in these high-risk patients which resulted in a significant decrease in sternal wound infections. Multi-variate analysis revealed that ITA grafting performed in the period of 1996–1999 was associated with a higher incidence of sternal infections. Hence in this series, eliminating high-risk patients, and not the technique of ITA harvesting, was responsible for the decrease in sternal wound infections. Raza et al. studied the incidence of DSWI in patients with SITA plus radial artery (RA) grafting vs. BITA grafting in over 1,300 patients with perioperative glycemic control (45). The incidence of DSWI was 1.4% in both groups. Vrancic et al. studied the incidence of DSWI in 3,118 patients with SITA and BITA grafting (46). The incidence of diabetes was identical in both groups (29%). The vast majority of surgery was performed off pump and tight glycemic control was practiced in all patients. The incidence of DSWI in all patients (diabetics and non-diabetics) was similar between SITA and BITA patients (1.5% vs. 1.9%; P=0.61). Although the incidence of DSWI was higher in diabetic patients, there was no difference between SITA vs. BITA grafting (3.1% vs. 3.7%; P=0.69). Despite the use of skeletonization, diabetes remained a risk factor for DSWI on multivariate analysis.
In summary, skeletonization resulted in a low incidence of DSWI for both SITA and BITA grafting. Nevertheless, DSWIs were not totally eliminated and was still higher in diabetic, obese, and COPD patients.
The incidence of DSWI in skeletonized vs. non-skeletonized ITAs
With the introduction of skeletonized ITAs and their perceived decrease in DSWIs with BITA grafting, several investigators sought to determine whether skeletonization resulted in a decrease in DSWIs compared to pedicled grafts, especially in high-risk patients. Peterson et al. studied the effects of skeletonization in 115 patients undergoing BITA grafting from 1990–2002 (47). The patients received a preoperative chlorhexidine wash and povidone-iodine soap scrub in the operating room. Prophylactic antibiotics (cefazolin or clindamycin) were administered perioperatively and bone wax was applied to the sternum. There was no protocol for glycemic control. In this patient cohort, 79 patients received a skeletonized BITA and 36 patients had pedicled grafts. The pedicle group had a significantly higher incidence of females and patients with COPD. The incidence of all sternal infections (5.1% vs. 22.2%; P=0.03) and DSWI (1.2% vs. 11.1%; P=0.03) were significantly lower in skeletonized BITA grafts. The overall incidence of sternal wound infections in skeletonized diabetic BITA patients was not different from pedicled BITA non-diabetic patients (4.8% vs. 4.2%; P=0.8). There were several limitations in this study. The study was retrospective and performed in an era where tight glycemic control was not practiced routinely. The pedicle group had a higher incidence of females and patients with COPD, two factors associated with increased DSWI. An overall incidence of sternal wound infections of 22% in the pedicle BITA group is higher than most centers in that era and suggests that factors other than skeletonization might have been responsible for these infections.
DePaulis et al. studied the effect of harvesting techniques on SSWI and DSWI in 450 patients with BITA grafting and 450 patients with pedicled SITA grafts (48). The study was retrospective, non-randomized, and without protocols for glycemic control. Bone wax was applied to the sternum. SITA patients had a lower incidence of DSWI than BITA patients (1.1% vs. 4.2%; P=0.004). In patients undergoing BITA grafting, there was no difference in the incidence of DSWI between skeletonized and pedicle grafts (3.3% vs. 4.7%; P=0.4). Pedicled SITA grafts have significantly less superficial infections than skeletonized or pedicled BITAs (4.8% vs. 7.8% vs. 12%; P=0.002). In patients with diabetes, the incidence of DSWI was similar between skeletonized SITA and skeletonized BITA grafts (1.6% vs. 3.5%; P=0.4), but significantly lower than pedicled BITA grafts (12.5%; P<0.01). Multivariate analyses demonstrated that pedicled BITA grafts were an independent risk factor for both SSWI and DSWI.
Calafiore et al. reported the results of SITA vs. BITA in diabetic patients where the ITA was skeletonized in 338 patients and harvested as a pedicle in 62 patients (49). The study was retrospective and non-randomized; however, strict glycemic control was practiced in all patients. Pedicled ITA grafts had a higher incidence of sternal wound infections in both SITA (6.7% vs. 1.8%; P=0.33) and in BITA patients (6.7% vs. 1.2%; P=0.20). However, due to the small sample size of pedicled grafts, the results were not statistically significant. Rubens et al. could find no difference in SSWI (3.4% skeletonized vs. 3.8% non-skeletonized; P=0.24) or DSWI (2.4% skeletonized vs. 2.5% non-skeletonized; P=0.27) in 1,500 CABG patients undergoing pedicle or skeletonized ITA grafts (50). Benedetto et al. studied the incidence of sternal wound infections in 3,102 patients enrolled in the Arterial Revascularization Trial (51). A pedicled BITA graft significantly increased the risk of any sternal wound infection (3.5% vs. 1.9%; P=0.0005) in diabetic and non-diabetic patients. When the analysis was restricted to only severe sternal wound infections, there was no increase in DSWI between pedicled BITA and pedicled SITA grafts. A skeletonized BITA resulted in an increase in DSWI compared to a skeletonized or pedicled SITA. Although a pedicled ITA was an independent risk factor for the development of any type of sternal wound infection, the mortality at 30 days was equivalent amongst all ITA groups: BITA, SITA—pedicled vs. skeletonized. This study was limited in that it was a subanalysis, non-randomized and underpowered to detect differences in DSWI amongst the various ITA groups. The majority of sternal infections were minor; only 3.6% were DSWI and only 1.2% required sternal reconstruction. Furthermore, no mention was made regarding glycemic control or the use of prophylactic antibiotics.
Since most studies regarding the impact of skeletonized vs. pedicled ITA grafts were underpowered to demonstrate significant differences in DSWI, several meta-analyses sought to more clearly define the significance of harvesting techniques on the incidence of sternal wound infections. Saso et al. found that skeletonization of ITA grafts decreased the incidence of all sternal infections in diabetic patients from 21.3% to 3.5% (52). Skeletonization of BITA grafts reduced the incidence of sternal wound infections from 11.7% to 2.96% in all patients and from 14.2% to 2.4% in diabetic patients. Hu et al. reviewed 22 trials between 1996–2010 involving 5,184 patients (53). Skeletonized grafts were longer in length and resulted in increased blood flow, but took significantly longer (P=0.008) to harvest. Skeletonized grafts were associated with significantly (P=0.033) less postoperative chest wall pain and dysesthesia, but following discharge, there was no difference in either of these parameters between skeletonized and pedicled grafts. The incidence of any sternal infection was significantly less in skeletonized ITAs (P=0.017). There were however, several limitations with this meta-analysis. Only 40% of the studies had BITA grafts. No mention was made of glycemic control or the use of prophylactic perioperative antibiotics. Variations were noted in techniques for both pedicled and skeletonized ITA harvesting, and the length of conduit that was dissected. Variations were also noted in the measurement of ITA flow and the use of papaverine and intraluminal dilation of the grafts. Only 9 of the trials reviewed were prospectively randomized. Deo et al. reviewed 126,235 patients from 10 observational and 1 randomized study (54). They found that pedicled BITA grafts significantly increased the incidence of DSWI from SITA grafts (3.1% vs. 1.6%; P<0.05) but there was no difference in DSWI between skeletonized BITA vs. skeletonized SITA grafts (0.42% vs. 2.09%; NS). As in other studies, the use of glycemic control was not mentioned. Furthermore, three studies did not specifically mention the technique used for BITA harvesting—it was assumed that unless specified, it was a pedicled ITA graft.
Discussion
Does BITA grafting increase DSWI, and should it only be performed in low risk patients? When utilizing the ITA, should it be routinely skeletonized? Unfortunately, the current literature does not allow us to conclusively answer these questions. The majority of studies are retrospective, observational, non-randomized, underpowered, and from different time periods, during which time there were significant changes in perioperative management that have impacted the incidence of DSWI, irrespective of the ITA harvesting techniques. Many studies did not specifically describe whether ITAs were or were not skeletonized, the specific techniques and types of cautery that was used, and the extent of the dissection. Often, no mention of glycemic control or protocols for the administration of perioperative antibiotics was made. The specific type of infection, SSWI vs. DSWI, was not always reported. The techniques used to close the sternum and the use of bone wax were not detailed. Finally, no mention was made of preoperative surveillance for staph colonization and whether topical antibiotics were applied to the sternal edges instead of bone wax.
The etiology of sternal infections is multi-factorial and requires a multi-faceted approach to prevent this complication. This is independent of the use of the ITA and the method by which it is harvested. The principles of preventing sternal wound infections were recently reviewed in the AATS guidelines (1). Preoperatively, all cardiac surgery patients should be screened for Staph species and mupirocin should be administered to all positive carriers. All distant extrathoracic infections should be treated prior to surgery. Patients should receive a chlorhexidine bath or shower to reduce skin bacterial counts. Strict glycemic control should be practiced in the preoperative, intraoperative, and for at least 24–48 hours postoperatively using IV insulin infusions to maintain serum glucose <180 mg/dL. This has independently been shown to decrease the incidence of sternal infections in CABG patients with ITA grafting (55-57). In those patients with COPD, an active smoking cessation program should be instituted to minimize coughing postoperatively to decrease the risk of sternal dehiscence. Perioperative prophylactic antibiotics should be instituted per the Society of Thoracic Surgeons (STS) guidelines (58,59). Topical antibiotics should be applied to the cut edges of the sternum after opening and prior to closing the sternotomy incision. Lazar et al. found that the application of topical vancomycin to the sternal edges in conjunction with perioperative antibiotics and tight glycemic control, resulted in total elimination of all sternal infections in 3,000 patients—both diabetic and non-diabetic in whom 65% had at least 1 ITA and 5% had BITA grafting of which only 25% had skeletonized grafts (60). Bone wax should be avoided since it acts as a foreign body, prevents bone union, and is an independent risk factor for both sternal dehiscence and wound infections (61). Every attempt should be made to open the sternum in the midline and use a Robicsek weave technique to stabilize the sternum if fractures occur during any ITA harvest. A figure-of-8 closing technique is preferable and wires should be placed as close as possible to the edge of the sternum to avoid injuring collateral vessels. Rigid sternal fixation with bands or plates should be considered in obese and COPD patients along with external chest support vests.
An example of the benefits of a multifaceted approach used to reduce sternal infections is provided by Kieser et al. (62). Over a 14-year period, multiple measures were instituted in 1,001 CABG patients in an attempt to decrease the incidence of DSWI. This included skeletonizing the ITA, avoiding bone wax, employing vancomycin paste on the sternal edges, using the harmonic scalpel to harvest the ITA, and applying a chlorhexidine alcohol skin prep and using iodine impregnated skin drapes. They found that the highest incidence of sternal wound infections occurred in obese, diabetic females despite skeletonizing all ITA grafts in these patients. While obese diabetic females represented only 13% of all CABG patients, they comprised 38% of all DSWIs, and were thus eliminated from BITA grafting. By instituting all of these measures, their incidence of DSWI fell from 3.1% to 0%. No one single factor was found to be responsible for this significant improvement. This lends further support to the concept that skeletonization can help to reduce, but by itself, does not eliminate DSWI. The etiology for DSWI is multifactorial and involves the institution of multiple therapeutic interventions as outlined in the AATS guidelines (1).
Is there a role for pedicled ITA grafts? Do they independently increase the incidence of DSWI, especially in BITA patients? Pedicled ITA grafts are easier to handle and can be harvested in less time than skeletonized grafts. Pedicled ITA grafts can still be successfully employed in CABG surgery. As our understanding of the blood and nerve supply of the sternum has improved, certain alterations during the harvesting of the pedicled ITA will help to minimize interruption of sternal blood flow and decrease sternal pain and dysesthesia. Sajja et al. studied the effects of preserving the pericardiacophrenic artery and dividing the pedicled ITA proximal to the distal ITA bifurcation (63). These maneuvers result in preservation of communicating musculophrenic branches and the superior epigastric branch, which also provides collaterals to the chest wall. The ITA was harvested as a pedicled graft from the takeoff of the subclavian vein to just proximal to the distal ITA bifurcation. All branch vessels were clipped as close as possible to the ITA. The study involved 3,072 diabetic and non-diabetic patients with SITA and BITA grafting—all of which were harvested as a pedicle graft. There was no difference in the incidence of DSWI amongst BITA diabetic patients, SITA diabetic patients, BITA non-diabetic patients and SITA non-diabetic patients (0.55% vs. 0.48% vs. 0.62% vs. 0.82%; P=0.83). Similarly, there was no difference in SSWI between the same groups (1.1% vs. 1.65% vs. 1.86% vs. 1.65%; P=0.62). On multivariate analysis, BITA harvesting and diabetes were not predictors of any sternal wound infections. This study shows that by modifying the pedicled ITA harvesting technique to spare the bifurcation of the distal ITA and preserve the pericardiacophrenic artery, the incidence of all sternal infections can be significantly reduced, even in diabetic and BITA grafted patients.
Choo et al. also sought to determine whether BITAs harvested as a pedicle graft would increase the risk of DSWI in a series of 207 patients, all with pedicled grafts (64). Diabetic patients comprised 71% of the series and 65% of diabetic patients received pedicled BITA grafts. The ITA was divided just proximal to the superior epigastric bifurcation and the dissection was performed using a harmonic scalpel (Ethicon Endo-Surgery, CVG, Cincinnati, Ohio, USA). Excessive bone wax was avoided, glycemic control was implemented, and all patients received prophylactic perioperative antibiotics. The incidence of DSWI was 0%. These two studies demonstrate that pedicled BITAs can be utilized during CABG surgery without any further increase in DSWI, even in diabetic patients. Important technical details in all harvesting techniques include clipping intercostal branches as close as possible to the ITA, preserving the proximal pericardiacophrenic and distal superior epigastric arteries, and avoidance of diathermy and cauterization, especially on the chest wall. The AATS guidelines should be followed to treat all staph nasal carriers, prophylactic perioperative antibiotic and tight glycemic protocols should be instituted, bone wax should be avoided, and topical antibiotics used instead. A midline sternotomy should be performed and all sternal fractures should be immediately stabilized to obtain optimal sternal closure.
When should the ITA be skeletonized? The decision to skeletonize the ITA should be made according to the surgeons’ preference, but there are instances when skeletonizing the ITA is preferable to a pedicled graft. In general, skeletonized ITAs are longer in length and are more likely to reach more distal targets on the posterior myocardium, especially in enlarged or hypertrophied hearts. Skeletonization makes it easier to perform sequential grafting, especially when constructing multiple side-to-side anastomoses. There is some evidence that skeletonization results in increased blood flow and is less likely to result in ITA spasm. This may be especially valuable when the ITA is used to provide the sole blood supply to large areas of the myocardium, especially during sequential grafting in hypertrophied hearts. This may decrease the incidence of postoperative low cardiac output syndrome.
Should the use of BITA grafts be curtailed to avoid DSWI? BITA grafting should be used in those patients in whom multiple arterial grafts have been shown to increase survival and decrease recurrent ischemic events. Although the 5-year results of the ART trial showed no difference in survival between SITA and BITA grafts, younger patients with less co-morbidities stand to gain the most from BITA grafting and it should not be avoided in this patient cohort. Furthermore, a longer period of time may be necessary to show the improved survival derived from BITA grafting. Patients who are at most risk for DSWI following BITA grafting are those in whom sternal healing would be compromised irrespective of the ITA harvesting technique. These are patients with underlying metabolic derangements, such as poorly controlled diabetes, frail patients with malnutrition and serum albumen levels <3.0 mg/dL and patients in whom postoperative sternal dehiscence is more likely due to physical factors such as obesity, COPD, smokers with persistent coughing and immobile patients in whom postoperative sternal precautions cannot be instituted. Finally, patients in whom a midline sternotomy was not performed have a higher incidence of postoperative sternal dehiscence and should not undergo BITA grafting. Since studies have shown that decreases in sternal blood flow are transient and occur mainly early in the postoperative period following BITA grafting, every effort should be made to optimize glycemic control, avoid bone wax and use topical antibiotics on the sternal edges and adhere to perioperative antibiotic protocols. This will ensure that the patient leaves the operating room with sternal closure to improve postoperative stability.
Conclusions
The development of DSWI following ITA grafting is multifactorial and independent of the harvesting technique. Skeletonization alone will not eliminate sternal wound infections. When utilizing pedicled ITA grafts, branch vessels should be clipped as close as possible to the ITA and the pericardiacophrenic artery and distal ITA bifurcation should be preserved. The use of a harmonic scalpel and low dose electrocautery is less likely to interrupt collateral blood flow and cause injury to the intercostal nerves. BITA grafting should be avoided in frail, malnourished, immobilized and poorly controlled diabetic patients, and in those in whom a midline sternotomy was not performed. Obese and COPD patients may benefit form postoperative sternal support vests. AATS guidelines should be closely followed to reduce sternal infections in all patients, irrespective of the use of ITA grafting and a particular harvesting technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lazar HL, VanderSalm T, Engelman R, et al. Expert Consensus Review: Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg 2016;152:962-72. [Crossref] [PubMed]
- D’Agostino RS, Jacobs JP, Badhwar V, et al. The STS adult cardiac surgery database: 2018 Update on outcomes and quality. Ann Thorac Surg 2018;105:15-23. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, Altman DG, et al. Effect of bilateral internal artery grafting on long-term survival---A meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Iribarne A, Goodney PP, Flores AM, et al. National Trends and Geographic Variation in Bilateral Internal Mammary Artery Use in the United States. Ann Thorac Surg 2017;104:1902-7. [Crossref] [PubMed]
- Berdajs D, Zund G, Turina MI, et al. Blood supply of the sternum and its importance in internal thoracic artery harvesting. Ann Thorac Surg 2006;81:2155-9. [Crossref] [PubMed]
- Arnold M. The surgical anatomy of sternal blood supply. J Thorac Cardiovasc Surg 1972;64:596-610. [PubMed]
- Cohen AJ, Lockman J, Lorberboym M, et al. Assessment of sternal vascularity with single photon emission computed tomography after harvesting of the internal thoracic artery. J Thorac Cardiovasc Surg 1999;118:496-502. [Crossref] [PubMed]
- Parish MA, Asai T, Grossi EA, et al. The effects of different techniques of internal mammary artery harvesting on sternal blood flow. J Thorac Cardiovasc Surg 1992;104:1303-7. [PubMed]
- Kamiya H, Akhyari P, Martens A, et al. Sternal microcirculation after skeletonized versus pedicled harvesting of the internal thoracic artery: A randomized study. J Thorac Cardiovasc Surg 2008;135:32-7. [Crossref] [PubMed]
- Carrier M, Gregoire J, Tronc F, et al. Effect of internal mammary artery dissection on sternal vascularization. Ann Thorac Surg 1992;53:115-9. [Crossref] [PubMed]
- Kouchoukos NT, Wareing TH, Murphy SF, et al. Risks of bilateral internal mammary bypass grafting. Ann Thorac Surg 1990;49:210-7. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179-86. [Crossref] [PubMed]
- Grossi EA, Esposito R, Harris LJ, et al. Sternal wound infections and the use of internal mammary artery grafts. J Thorac Cardiovasc Surg 1991;102:342-6. [PubMed]
- Nakano J, Okabayashi H, Hanuyu M, et al. Risk factors for wound infection after off-pump CABG: Should bilateral internal thoracic arteries be harvested in patients with diabetes? J Thorac Cardiovasc Surg 2008;135:540-5. [Crossref] [PubMed]
- Dai C, Lu Z, Zhu H, et al. Bilateral internal mammary artery grafting and risk of sternal wound infection: evidence from observational studies. Ann Thorac Surg 2013;95:1938-45. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial of bilateral vs single internal thoracic artery grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Gansera B, Schmidter F, Gillrath G, et al. Does bilateral internal thoracic artery grafting increase perioperative complications? Outcome of 4462 patients with bilateral versus 4204 patients with single internal mammary artery bypass. Eur J Cardiothorac Surg 2006;30:318-23. [Crossref] [PubMed]
- Elmistekawy EM, Gawad N, Bourke M, et al. Is bilateral internal thoracic artery use safe in the elderly? J Card Surg 2012;27:1-5. [Crossref] [PubMed]
- Walkes JC, Earle N, Reardon MJ, et al. Outcomes in single vs bilateral internal thoracic artery grafting in coronary artery bypass grafting. Curr Opin Cardiol 2002;17:598-601. [Crossref] [PubMed]
- Puskas JD, Sadiq A, Vassiliades TA, et al. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann Thorac Surg 2012;94:710-5; discussion 715-6. [Crossref] [PubMed]
- Grau JB, Ferrari G, Mak AW, et al. Propensity matched analysis of bilateral internal mammary artery versus single left internal mammary artery grafting at 17-year follow-up: validation of a contemporary surgical experience. Eur J Cardiothorac Surg 2012;41:770-5; discussion 776. [Crossref] [PubMed]
- Galbut DL, Kurlansky PA, Tradd EA, et al. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: a propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg 2012;143:844-53.e4. [Crossref] [PubMed]
- Ioannidis JP, Galanos O, Katritsis D, et al. Early mortality and morbidity of bilateral versus single internal thoracic artery revascularization: propensity and risk modeling. J Am Coll Cardiol 2001;37:521-8. [Crossref] [PubMed]
- Kelly R, Buth KJ, Legare JF. Bilateral internal thoracic artery grafting is superior to other forms of multiple arterial grafting in providing survival benefit after CABG. J Thorac Cardiovasc Surg 2012;144:1408-15. [Crossref] [PubMed]
- Itagaki S, Cavallero P, Adams DH, et al. Bilateral internal thoracic artery grafts, mortality and morbidity: an analysis of 1,526,360 coronary bypass operations. Heart 2013;99:849-53. [Crossref] [PubMed]
- Stamou SC, Nussbaum M, Stiegel RM, et al. Effect of body mass index on outcomes after cardiac surgery: Is there an obesity paradox? Ann Thorac Surg 2011;91:42-7. [Crossref] [PubMed]
- Engel AM, McDonough S, Smith JM. Does an obese body mass index affect hospital outcomes after coronary artery bypass graft surgery. Ann Thorac Surg 2009;88:1793-800. [Crossref] [PubMed]
- Reeves BC, Ascione R, Chamberlain MH, et al. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol 2003;42:668-76. [Crossref] [PubMed]
- Hirotani T, Kameda T, Kumamoto T, et al. Effects of CABG using internal mammary arteries for diabetic patients. J Am Coll Cardiol 1999;34:532-8. [Crossref] [PubMed]
- LaPar DJ, Crosby IK, Rich JB, et al. Bilateral internal thoracic artery use for CABG remains underutilized: A propensity –matched multi-institutional analysis. Ann Thorac Surg 2015;100:8-14. [Crossref] [PubMed]
- Keeley SB. The skeletonized internal mammary artery. Ann Thorac Surg 1987;44:324-5. [Crossref] [PubMed]
- Deja MA, Wos R, Golba KS, et al. Intraoperative and laboratory evaluation of skeletonized versus pedicled internal thoracic artery. Ann Thorac Surg 1999;68:2164-8. [Crossref] [PubMed]
- Takami Y, Ina H. Effects of skeletonization on intraoperative flow and anastomosis diameter of the internal thoracic arteries in coronary artery bypass graft surgery. Ann Thorac Surg 2002;73:1441-5. [Crossref] [PubMed]
- Mailis A, Umana M, Feindel CM. Anterior intercostal nerve damage after CABG surgery with the use of the internal mammary artery graft. Ann Thorac Surg 2000;69:1455-8. [Crossref] [PubMed]
- Mueller XM, Tinguely F, Tevaearai HT, et al. Pain pattern and left internal thoracic artery grafting. Ann Thorac Surg 2000;70:2045-9. [Crossref] [PubMed]
- Boodhwani M, Lam BK, Nathan HJ, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766-73. [Crossref] [PubMed]
- Markman PL, Rowland MA, Leong JY, et al. Skeletonized internal thoracic artery harvesting reduces chest wall dysesthesia after coronary bypass surgery. J Thorac Cardiovasc Surg 2010;139:674-9. [Crossref] [PubMed]
- Gaudino M, Toesca A, Nori SL, et al. Effect of skeletonization of the internal thoracic artery on vessel wall integrity. Ann Thorac Surg 1999;68:1623-7. [Crossref] [PubMed]
- Gaudino M, Trani C, Glieca F, et al. Early vasoactive profile of skeletonized versus pedicled ITA grafts. J Thorac Cardiovasc Surg 2003;125:638-41. [Crossref] [PubMed]
- Calafiore AM, Vitolla G, Iaco AL, et al. Bilateral internal thoracic artery grafting: midterm results of pedicled vs skeletonized conduits. Ann Thorac Surg 1999;67:1637-42. [Crossref] [PubMed]
- Bical O, Braunberger E, Fischer M, et al. Bilateral skeletonized mammary artery grafting: experience with 560 consecutive patients. Eur J Cardiothorac Surg 1996;10:971-5. [Crossref] [PubMed]
- Sofer D, Gurevitch J, Shapira I, et al. Sternal wound infections in patients after coronary artery bypass grafting using bilateral skeletonized internal mammary arteries. Ann Surg 1999;229:585-90. [Crossref] [PubMed]
- Endo M, Tomizawa Y, Nishida H. Bilateral vs single internal mammary artery revascularization in patients with diabetes. Circulation 2003;108:1343-9. [Crossref] [PubMed]
- Hemo E, Mohr R, Uretzky G, et al. Long-term outcomes of patients with diabetes receiving bilateral internal thoracic artery grafts. J Thorac Cardiovasc Surg 2013;146:586-92. [Crossref] [PubMed]
- Raza S, Blackstone EH, Houghtaling PL, et al. Similar outcomes in diabetic patients after coronary artery bypass grafting with single internal thoracic artery plus radial artery grafting and bilateral internal thoracic artery. Ann Thorac Surg 2017;104:1923-32. [Crossref] [PubMed]
- Vrancic JM, Piccinini F, Camporrotondo M, et al. Bilateral internal thoracic artery grafting increases mediastinitis: Myth or fact. Ann Thorac Surg 2017;103:834-9. [Crossref] [PubMed]
- Peterson MD, Borger MA, Rao V, et al. Skeletonization of bilateral internal thoracic arteries lowers the risk of sternal infections in patients with diabetes. J Thorac Cardiovasc Surg 2003;126:1314-9. [Crossref] [PubMed]
- De Paulis R, deNotaris S, Scaffa R, et al. The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: The role of skeletonization. J Thorac Cardiovasc Surg 2005;129:536-43. [Crossref] [PubMed]
- Calafiore AM, DiMauro M, Giammarco GD, et al. Single versus bilateral internal mammary artery for isolated first myocardial revascularization in multivessel disease: Long-term clinical results in medically treated diabetic patients. Ann Thorac Surg 2005;80:888-95. [Crossref] [PubMed]
- Rubens FD, Chen L, Bourke M. Assessment of the association of bilateral internal thoracic artery skeletonization and superficial infection after coronary artery bypass grafting. Ann Thorac Surg 2016;101:1677-82. [Crossref] [PubMed]
- Benedetto U, Altman DG, Gerry S, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152:270-6. [Crossref] [PubMed]
- Saso S, James D, Vecht JA, et al. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg 2010;89:661-70. [Crossref] [PubMed]
- Hu X, Zhao Q. Skeletonized internal thoracic artery harvest improves prognosis in high-risk population after coronary artery bypass for good quality grafts. Ann Thorac Surg 2011;92:48-58. [Crossref] [PubMed]
- Deo SV, Shah IK, Dunlay SM, et al. Bilateral internal thoracic artery harvest and deep sternal wound infection in diabetic patients. Ann Thorac Surg 2013;95:862-9. [Crossref] [PubMed]
- Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109:1497-502. [Crossref] [PubMed]
- Zerr KJ, Furnary AP, Grukemeier GL, et al. Glucose control lowers the risk of wound infection in diabetes after open chest operations. Ann Thorac Surg 1997;63:356-61. [Crossref] [PubMed]
- Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67:352-60; discussion 360-2. [Crossref] [PubMed]
- Engelman R, Shahian D, Shemin R, et al. The Society of Thoracic Surgeons Practice Guidelines Series: Antibiotic prophylaxis in cardiac surgery. Part II: Antibiotic choice. Ann Thorac Surg 2007;83:1569-76. [Crossref] [PubMed]
- Edwards FH, Engelman RM, Houck P, et al. The Society of Thoracic Surgeons Practice Guidelines Series: Part 1: Duration. Ann Thorac Surg 2006;81:397-404. [Crossref] [PubMed]
- Lazar HL, Ketchedjian A, Haime M, et al. Topical vancomycin in combination with perioperative antibiotics and tight glycemic control helps to eliminate sternal infections. J Thorac Cardiovasc Surg 2014;148:1035-8; 1038-40.
- Prziborowski J, Hartrumpf M, Stock VA, et al. Is bone wax safe and does it help? Ann Thorac Surg 2008;85:1002-6. [Crossref] [PubMed]
- Kieser TM, Rose MS, Aluthman V, et al. Toward zero: deep sternal wound infection after 1001 consecutive coronary artery bypass procedures using arterial grafts: Implications for diabetic patients. J Thorac Cardiovasc Surg 2014;148:1887-95. [Crossref] [PubMed]
- Sajja LR, Mannam G, Bandu SBR, et al. Reduction of sternal wound infections in diabetic patients undergoing bilateral internal thoracic artery harvest technique. J Thorac Cardiovasc Surg 2012;144:480-5. [Crossref] [PubMed]
- Choo SJ, Lee SK, Chung SW, et al. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Yonsei Med J 2009;50:78-82. [Crossref] [PubMed]





