Infective valve endocarditis after transcatheter aortic valve implantation—a dangerous liaison
Introduction
Infective endocarditis (IE) is a rare disease in the general population (1), but the annual incidence is markedly increased in patients with previous cardiac valve surgery, which impacts 4,000–6,000 cases per million patients (2). Due to the recent development of transcatheter aortic valve implantation (TAVI), our knowledge about TAVI-IE is still limited, in particular, in comparison to IE developing after surgical aortic valve replacement (SAVR-IE). Initial data on TAVI-IE are concerning, since it is difficult to diagnose and prognosis is dismal. This Editorial will summarize our current knowledge concerning diagnosis, treatment and prevention of TAVI-IE.
Diagnosing TAVI-IE
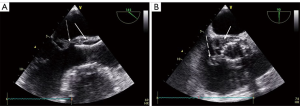
As in SAVR-IE, diagnosis is more difficult in TAVI-IE than in native valve IE, with a lower diagnostic value of echocardiography (3) except in massive findings as shown in the Figure 1. Previous registries have shown negative echocardiographic imaging in about 30% of patients with TAVI-IE (4,5), but those patients, compared to patients with a positive imaging, had a comparably worse prognosis (4). Different echocardiographic patterns have been described in TAVI-IE, including an obstructive pattern with leaflet thickening and high transvalvular gradient as the only abnormality at early stages (6). Other imaging techniques, such as multislice computed tomography (CT) and 18F-FDG (18F-fluorodeoxyglucose) positron emission tomography (PET)/CT are useful in the setting of suspected prosthetic valve IE and have been included in the new European Society of Cardiology (ESC) criteria (1). However, PET/CT and cardiac CT have not been specifically studied in TAVI-IE and the value of the combination of these techniques in a multimodality approach is unknown. This uncertainty explains why the value of the Duke criteria (1) is indefinite in this population and many patients are classified as “possible” IE due to negative imaging. Therefore, prompt diagnosis and treatment is necessary in cases with high clinical suspicion for IE, in particular if continuous bacteremia with the two most common agents Staphylococcus and Enterococcus is evident (4,5).
Incidence of TAVI-IE
Despite all the diagnostic difficulties, the incidence of TAVI-IE has been reported to be about 1.1% to 1.8% per patient-year (4,5). Two recent cohort studies revealed that there is no significant difference in the incidence of TAVI-IE (1.6–1.7% per patient-year) compared to SAVR-IE (1.2–1.9% per patient-year) up to five years of follow-up (7,8).
Treating TAVI-IE
Early surgery is recommended in complicated cases of IE, including those with high risk of embolism, perivalvular complications and congestive heart failure. Unfortunately, these recommendations cannot be applied in most of the patients currently treated by TAVI that develop IE, as contraindications to surgery frequently exist in this high-risk population. In the largest registry, surgery was performed in only 14.8% of patients with TAVI-IE despite 81.2% having at least 1 indication for surgery, according to current guidelines (5) and cardiac surgery was not associated with a reduced risk of in-hospital death. This very low rate of valve surgery is likely due to the high surgical risk of such patients, in addition to the potential technical difficulties like removing a stent frame adherent to the aorta. Against this background, it is not surprising that TAVI-IE is associated with a devastating in-hospital and mid-term mortality ranging from 34–63.6% and 66.7–74.5%, respectively (4,5).
In a recent analysis, we tried to gain more information on the role of cardiac surgery in patients with TAVI-IE (9). In a series of 64 patients with TAVI-IE, 20 were treated by surgery. The 44 patients treated by antibiotic therapy only were older (P=0.006), had higher Society of Thoracic Surgeons scores (P=0.029), and more often had severe chronic kidney disease (P=0.037). One-year mortality was not different between groups, but the complication rate was higher in the surgical group (P=0.024). The results were not significantly different after manual matching comparing 20 patients treated by antibiotics only vs. 20 patients treated by cardiac surgery (and antibiotics). Due to several limitations (retrospective, observational, nonrandomized study in a small patient population treated at a single centre, potential imaging selection bias), we are unable to conclude whether surgery is better when compared to medical therapy alone in these patients. However, this is also true for any prosthetic valve IE treatment since our guideline recommendations are based on registries (10) without having data derived from randomized controlled trials. In the era of expanding indications for TAVI, all efforts should be made to create multicenter, prospective registries and studies, if possible randomized, to assess the real role of surgery in these patients.
Preventing TAVI-IE
If treatment of TAVI-IE is so difficult, the best approach is to prevent it. Although guidelines recommend antibiotic prophylaxis for patients with any prosthetic valve in case of dental procedures with increased risk for bacteremia (1), Streptococcal infection is very rare in TAVI-IE. Conversely, Staphylococcal and, probably more important, Enterococcal infections are most frequent in this population (4,5). This underscores the crucial need to focus on prevention rather than prophylaxis in those patients who have high exposure to healthcare procedures, older age and foreign material (4,5), including aseptic measures during any invasive procedure and use of antibiotics potentially adapted to these microorganisms for prophylaxis during TAVI procedures (5).
Conclusions
Further studies are needed to provide clear information to the clinician about the optimal use of new imaging techniques to diagnose TAVI-IE and the best way to treat it when diagnosis is definite. We should recognize that factors other than surgery mainly influence outcome in patients with TAVI-IE, including comorbidity, frailty, heart failure, renal failure (5) and disease characteristics (9). We do not currently have enough published data on the role of cardiac surgery in prosthetic valve IE and particularly TAVI-IE. For this reason, the treatment decision needs to be individualized depending on the clinical status, operative risk and comorbidities. More importantly, because both diagnosis and treatment are particularly difficult for patients with suspected TAVI-IE, these patients should be referred to reference centers and the “endocarditis team” should make any decision as outlined in current guidelines (1).
Acknowledgments
None.
Footnote
Conflicts of Interest: Norman Mangner reports speaker’s honoraria from Edwards, Medtronic, Novartis, Sanofi Genzyme and Astra Zeneca, consultant honoraria from Biotronik, outside the submitted work. Axel Linke reports grants and personal fees from Medtronic, personal fees from St. Jude Medical, grants from Claret Medical, personal fees and other from Claret Medical, personal fees from Boston Scientific, personal fees from Bard, personal fees from Edwards, outside the submitted work.
References
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Thornhill MH, Jones S, Prendergast B, et al. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. Eur Heart J 2018;39:586-95. [Crossref] [PubMed]
- Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007;297:1354-61. [Crossref] [PubMed]
- Mangner N, Woitek F, Haussig S, et al. Incidence, Predictors, and Outcome of Patients Developing Infective Endocarditis Following Transfemoral Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;67:2907-8. [Crossref] [PubMed]
- Regueiro A, Linke A, Latib A, et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016;316:1083-92. [Crossref] [PubMed]
- Salaun E, Sportouch L, Barral PA, et al. Diagnosis of Infective Endocarditis After TAVR: Value of a Multimodality Imaging Approach. JACC Cardiovasc Imaging 2018;11:143-6. [Crossref] [PubMed]
- Butt JH, Ihlemann N, De BO, et al. Long-Term Risk of Infective Endocarditis After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2019;73:1646-55. [Crossref] [PubMed]
- Kolte D, Goldsweig A, Kennedy KF, et al. Comparison of Incidence, Predictors, and Outcomes of Early Infective Endocarditis after Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in the United States. Am J Cardiol 2018;122:2112-9. [Crossref] [PubMed]
- Mangner N, Leontyev S, Woitek FJ, et al. Cardiac Surgery Compared With Antibiotics Only in Patients Developing Infective Endocarditis After Transcatheter Aortic Valve Replacement. J Am Heart Assoc 2018;7:e010027. [Crossref] [PubMed]
- Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013;173:1495-504. [Crossref] [PubMed]






