Ex-vivo lung perfusion versus standard protocol lung transplantation—mid-term survival and meta-analysis
Introduction
While lung transplantation (LTx) for patients with end-stage pulmonary disease can be a life-saving measure, the scarcity of suitable donor lungs results in up to 30% of these patients dying while on the waiting list (1,2). Obtaining donor organs for LTx is particularly problematic, with donors of at least one other solid organ only having suitable lungs in 15–30% of cases according to current assessment criteria (3-5). End-of-life care, be it in the intensive care unit or otherwise, often results in damage to potential grafts and even under optimal donor circumstances, brain death still has the deleterious effect of causing neurogenic edema and a cytokine storm-induced inflammatory response (6).
Standard transplant protocols developed and refined since the 1960’s involve infusion of the donor lung with a specially formulated perfusate before inflation of the lung, stapling of the trachea, and cold preservation until transplantation (7). In contrast, ex-vivo lung perfusion (EVLP) involves continuous ventilation and perfusion of donor lungs, offering the potential to both extend the ability to functionally assess grafts, as well as recondition them to a transplant-suitable standard, thereby expanding the available pool of grafts (8). EVLP can be administered via several different established protocols which vary perfusate composition, flow, temperature, pressure, and ventilation, with off-the-shelf EVLP equipment such as the “XVIVO Perfusion System (XPS)”, “Organ Care System (OCS)”, and “Vivoline LS-1” now on the market (1).
While extended-criteria lungs have been shown to have comparable follow-up survival outcomes to standard criteria lungs using standard lung transplant methods, the use of EVLP to further expand the donor pool raises the question of EVLP recipient long-term outcomes (3,8,9). The objective of this meta-analysis was to aggregate mid- to long-term survival data and available post-operative outcomes from studies comparing LTx recipients who received EVLP treated grafts to those receiving standard protocol (cold-preservation) grafts.
Methods
Literature search
Ten medical literature databases were queried from their dates of inception to August 2019. These included Medline, Embase, PubMed, and the Ovid “Evidence-Based Medicine Reviews” collection, which includes the Cochrane databases, as well as national college and government repositories. A broad search strategy was deliberately used, using terms “ex-vivo lung perfusion” (as a whole term and individually) and “EVLP”.
Two independent researchers (P.F. and B.M.) screened reference list results and full texts, with inclusion at each stage determined by consensus with the senior researcher (A.C.). Studies were included if they were comparative studies reporting primary mid- to long-term outcome data for recipients after lung transplant using standard protocol or EVLP. Studies needed to include at least five transplant recipients per arm. Non-comparative studies, animal studies, case reports, conference abstracts, reviews, and editorials were excluded. Where duplicate series exist, the study containing the most complete and up-to-date data was retained. The reference list of all included studies was examined to identify further articles meeting the inclusion criteria.
The primary endpoint was overall Kaplan-Meier survival reported to at least 6-months. Secondary endpoints were determined as those reported in at least half of included studies and included 30-day mortality, post-operative graft dysfunction grade 3 at 72 h, intensive care unit length of stay (LOS), and hospital LOS.
Quality analysis
A 19-point metric adopted from the Canadian Institute of Health Economics was used to assess included studies’ quality. This tool was designed with a Delphi methodology of health research stakeholders to evaluate the quality of case series, with examined domains including the number of centers data was collected from, study design, completeness of recipient and donor baseline data reporting, completeness of intraoperative and post-operative outcome reporting, and potential indicators of bias or conflict (Table S1). Total scores for each study were tallied to determine quality strata, with studies scoring below 12 deemed standard quality, 12 to 14 moderate quality, and 14 to 19 high quality.

Full table
Statistical analysis
Data extraction was performed by two independent researchers (P.F. and B.M.) with data checking and validation by the senior researcher (A.C.). Where data was expressed as median and range or interquartile range, it was converted to mean and standard deviation using statistical methods to facilitate pooling (10,11). Furthermore, where no standard deviation or range was provided, a sample value was imputed as the mean of other provided values (12,13). Pooling was performed using meta-analysis of proportions or means. Differences in baseline data and outcomes were summarized as relative risk (RR) and mean difference (MD) for proportion and continuous data, respectively, with 95% confidence intervals (95% CI) provided. A random effects model was applied for all analyses to account for between-study variance due to recipient and donor selection, procedural, and care differences not accounted for in institutional series. Studies with zero-event outcomes in both arms were not weighted in meta-analysis.
Survival data was aggregated using the method for secondary survival analysis developed by Guyot and colleagues (14). This approach imputes individual patient time-to-event data, taking digitized Kaplan-Meier survival curves (Engauge Digitizer, Mark Mitchell, GitHub) and patient number-at-risk data as inputs. The imputed individual patient data is then pooled as an overall cohort and aggregated survival curves generated. Hazard ratio (HR) between EVLP and standard treatment protocol is calculated from Kaplan-Meier data using a Cox proportional hazard model (15). Proportionality was tested with a Schoenfeld residual test.
Publication bias was examined with funnel plots and also by Egger’s test for study endpoints. Heterogeneity amongst studies were assessed using the I2 statistic, with consideration of I2 confidence intervals (16). I2 thresholds of 0–49%, 50–74%, and ≥75% were considered as low, moderate, and high heterogeneity, respectively (17). Potential sources of heterogeneity and inconsistency of treatment effect were identified and explored with the aid of leave-one-out sensitivity analyses.
Two-tailed P values less than 0.05 were deemed as significant. All statistics were performed with R (R foundation for statistical computing, Vienna, Austria).
Results
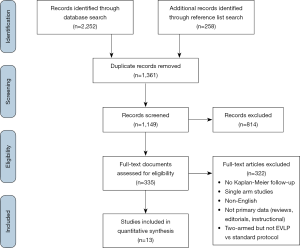
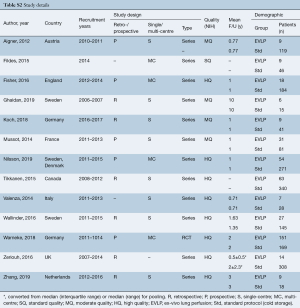
Literature search identified 2,252 references with 174 full-text articles screened for inclusion. Reference list search of initially included articles led to the identification of one additional study. In all, 13 studies were included for analysis (PRISMA diagram provided in Figure S1) (4,5,8,18-27). One randomized controlled trial (RCT) was included (27), while four studies were prospective, non-randomized trials (18,20-22), six were retrospective series (4,5,8,23,25,26), and two were series with their design not fully described (19,24). One study was rated as standard quality, four as moderate quality, and eight as high quality (Table S2). The majority of studies had small EVLP cohorts, with a median cohort size of 14 patients, and the largest EVLP cohort of 151 patients contained in the RCT by Warnecke et al. All but one study were from European centres.
Full table
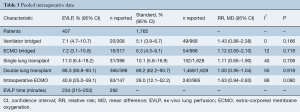
The thirteen comparative studies contained in-total 2,172 lung transplant recipients, with 1,765 transplanted using standard/cold storage protocol and 407 transplanted with EVLP lungs. Where reported, the mean follow-up ranged from 0.7–10 years, with a median of 1-year follow-up.
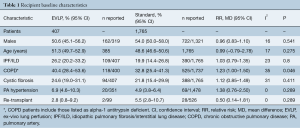
The mean age of EVLP lung transplant recipients was 51.3 years (95% CI: 49.7–52.9; n=385), with 53.3% males (95% CI: 50.0–56.5; n=884/1,640), while standard protocol lung transplant recipients had a mean age of 48.6 years (95% CI: 46.6–50.6, n=1,765), with 54.0% males (95% CI: 50.0–58.0, n=722/1,321). The pooled recipient cohorts differed only in that COPD was more prevalent in the EVLP recipients (40.4% vs. 32.8%, P=0.046). Recipient baseline details and risk are summarized in Table 1 and further detailed in Table S3.
Full table
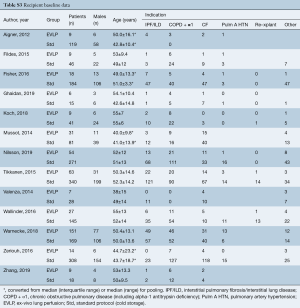
Full table
Despite high heterogeneity for some patient/donor baseline parameters, baseline data sensitivity analysis showed these were often due to factors such as inter-study variation in the number of DCD donors or donor PaO2/FiO2 ratio. Reasons for study exclusion were not identified and sensitivity analysis of study endpoints did not demonstrate individual studies changing overall findings of significance (with the exception of 30-day mortality).
Overall there were 2,178 lung donors, with 413 EVLP and 1,765 standard protocol donors. While not detailed across all studies, the majority of all grafts were from brain-dead donors (DBD, 88.0%; 95% CI: 80.4–93.0%). Mean age of EVLP donors was 47.3 years (95% CI: 44.5–50.1; n=359) and standard donors, 45.6 years (95% CI: 43.4–47.8; n=1,360). As expected with EVLP’s application for graft reconditioning, EVLP donors had significantly greater rates of abnormal chest X-ray [62.0%; (95% CI: 48.2–74.1%), versus 36.6% (95% CI: 25.6–49.2%), P=0.011], and poorer PaO2/FiO2 ratio [287 mmHg (95% CI: 217–358) versus 439 mmHg (95% CI: 427–451), P<0.001]. This difference in PaO2/FiO2 was further accentuated when the pooled PaO2/FiO2 included only studies that provided pre-EVLP values of PaO2/FiO2 (excluding Warnecke et al.): EVLP 272 mmHg (95% CI: 218–326, n=190), versus, standard 440 mmHg (95% CI: 426–454, n=1,126), MD −154 mmHg (95% CI: −214 – −94.3], I2=95%, P<0.001). Pooled donor baseline details are provided in Table 2 and per-study data in Table S4.
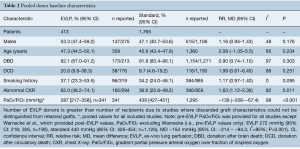
Full table
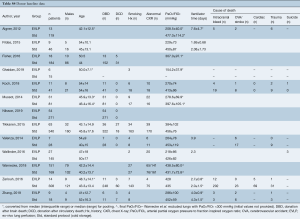
Full table
The mean EVLP time was 234 min (95% CI: 215−253, n=294). EVLP was performed using the proprietary XVIVO system (XVIVO, Denver, CO, USA) in five studies, Vivoline LS-1 (Vivoline Medical AB, Lund, Sweden) in three studies, Organ Care System (OCS-TransMedics Inc., Boston, MA, USA) in two studies, and not-fully described or administered with custom circuits in four studies (Table S5). The majority of patients received double-lung transplants. Intraoperative parameters were similar between EVLP and standard protocol groups (Table 3).
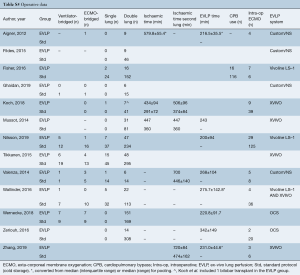
Full table
Full table
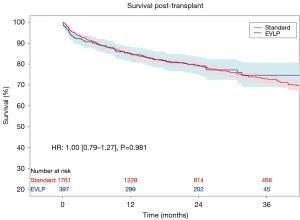
The primary outcome of Kaplan-Meier survival was reported from 12 to 120 months. Survival data in the single RCT was only available for the per-protocol, rather than the intention-to-treat, population, hence, the number at risk is reduced from the total number of patients reported for baseline and intraoperative data (27). Aggregated survival for individual patient data demonstrated almost identical survival profiles for both EVLP and standard/cold storage protocol lung transplant recipients. Survival at 12, 24, and 36 months for the EVLP cohort was 84%, 79%, and 74%, respectively, compared to 85%, 79%, and 73%, for the same time periods in the standard protocol cohort. Cox proportional hazard analysis demonstrated a hazard ratio of 1.00 (95% CI: 0.79–1.27; P=0.981; Figure 1).
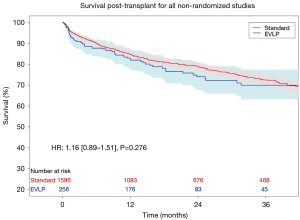
Leave-one-out analysis of Kaplan-Meier data was conducted to examine the sensitivity of Kaplan-Meier and hazard ratio results to individual patient data from each study. No single study was found to significantly influence survival outcomes. The aggregated survival curves from the non-randomized studies (and no matching between arm— excluding Warnecke et al.) is provided in Figure S2 (HR 1.16; 95% CI: 0.89–1.51; P=0.276).
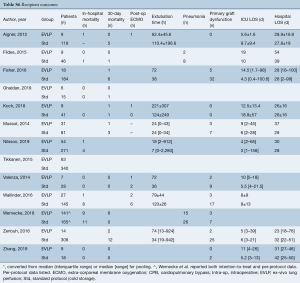
Operative outcomes were inconsistently reported across included studies, with only 30-day mortality, primary graft dysfunction grade 3 at 72 h (PGD3), extubation time, ICU LOS, and hospital LOS reported for the majority of studies. The 30-day mortality was similar between EVLP and standard cohorts, RR 2.04 (95% CI: 0.88–4.72, I2=0%, P=0.095), however, this result was obtained after exclusion of one particular study (27), which detailed each early death as non-EVLP related (due to iatrogenic surgical complications, patients’ compliance with medications, and patients’ cardiac risk factors). Including all studies gave a significant 30-day mortality result for EVLP: RR 2.39 (95% CI: 1.07–5.35, I2=0%, P=0.034). Primary graft dysfunction grade 3 at 72 hours did not reach significance (RR 1.15; 95% CI: 0.69–1.89, I2=0%, P=0.592). While continuous outcomes such as extubation time, intensive care unit, and hospital LOS were widely reported, heterogeneity of reporting and non-availability of standard deviation data meant the majority of data required imputation and multiple assumptions to allow pooling, and hence, it was not conducted. Per-study outcome data are detailed in Table S6, and pooled secondary outcomes in Table 4 and Figures 2,3.
Full table

Full table
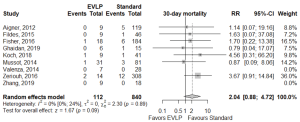
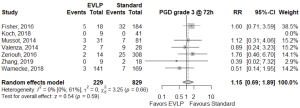
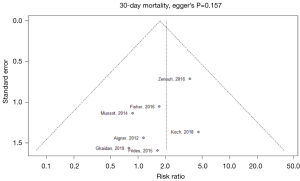
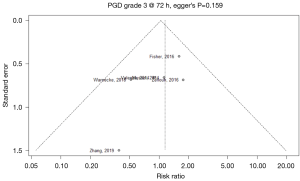
Publication bias in meta-analysed endpoints was not identified from visual inspection of funnel plots or application of Egger’s test (Figures S3,S4). Point values of heterogeneity for secondary outcomes remained at zero (I2=0%) for the sensitivity analysis of secondary outcomes.
Discussion
This meta-analysis combined results from 13 different studies detailing mid- to long-term survival data to demonstrate that there was no significant survival difference for transplant recipients receiving donor lungs treated with EVLP versus standard/cold storage protocol lungs. Neither 30-day mortality or grade 3 primary graft dysfunction at 72 h post-transplant (PGD3 at 72 h) were found to differ significantly between EVLP and standard cohorts.
The direction of primary and secondary outcomes of this meta-analysis were found to be concordant with all included studies, with the exception of the INSPIRE RCT, which identified a significantly greater 30-day mortality in the EVLP group but also a significant reduction in PGD3 at 72 h for EVLP patients. The authors of that study accounted for the significant 30-day mortality signal as non-EVLP related (due to iatrogenic surgical complications, patients’ compliance with medications, and patients’ cardiac risk factors) and this led to that outcome’s exclusion from this meta-analysis (27).
The PaO2/FiO2 threshold for acceptance of donor grafts varies between centres (22), as well as how this value is measured (for example, variations in time points, positive end expiratory pressure). However, this was not shown to significantly affect overall survival outcomes between EVLP and standard protocol lungs at a meta-analysis level, nor within included studies, which included donor lungs with pre-EVLP pooled mean PaO2/FiO2 value of 272 mmHg [and as low as 150 mmHg (4)]. Collectively, this information could be used to shape EVLP donor acceptance criteria and potentially further increase the number of donor lungs accepted for EVLP, for example from DCD donors, who typically have worse PaO2 values (4,23,25). The divergent but statistically non-significant survival curves evidenced with the removal of the RCT (Figure S2) likely reflect the fact that the INSPIRE RCT made up a large proportion of the pooled EVLP cohort and required both EVLP and standard donor groups to have similarly high PaO2/FiO2 ratios for acceptance. This was in contrast to the other included studies, where EVLP was used for reconditioning grafts with poorer pre-EVLP PaO2/FiO2 ratios.
PGD3 at 72 hours has been found to correlate with increased 30-day, 90-day, and 1-year mortality (26,28), as well as chronic lung allograft dysfunction (27). While the reduction in PGD3 at 72 h was only significant in the INSPIRE RCT, a reduced incidence of PGD3 at 72 h in EVLP transplant recipients was a finding shared by several other included studies (5,18,26). Though these studies’ findings on PGD3 did not reach significance and had smaller cohorts than the RCT, they all noted an absence of PGD3 events in their EVLP recipients, and other non-zero event studies similarly noted non-significant lower incidences. This was the case despite a worse PaO2/FiO2 in the meta-analysis EVLP donor group and in individual studies, where poorer PaO2/FiO2 donor lungs were accepted for EVLP treatment. Unlike other included studies, the INSPIRE RCT was exceptional in its exclusion of donors with PaO2/FiO2 <300 mmHg for both EVLP and standard groups. Although PGD3 was a secondary outcome in this meta-analysis, it’s non-significant difference between EVLP and standard groups in combination with the non-significant survival difference at follow-up is potentially valuable, as it adds weight to the restorative ability of EVLP and its potential for good long-term outcomes.
Other secondary outcomes examined and commented upon in many studies were post-transplant extubation time and ICU length of stay, with concerns that using high-risk lungs for EVLP may lead to an increase in these parameters and their associated risks (8,23). Pooling of these continuous outcomes was precluded by heterogeneity in reporting and a need to impute data for the majority of included studies. While the limitations of vote counting in meta-analysis are acknowledged (29), only one of thirteen included studies found significantly longer extubation times and ICU length of stay for EVLP (22).
While the constituent studies of this meta-analysis applied varying EVLP protocols and methodologies, it is clear that EVLP provides the ability to expand the pool of available donor lungs by reconditioning and reassessing lungs not considered suitable under standard criteria lung transplant. Using marginal lungs under extended-criteria for standard protocol lung transplant has been reported as doubling utilization rates to around 30–40% (30,31). Using EVLP however, studies in the present analysis reported conversion rates from EVLP evaluated lungs to transplant ranging from 34% to 97% (8,20,21,23,24,26). This allowed increased donor utilization, ranging from 33% to 50% (8,22,23). Along with the non-significant differences in outcomes found in this meta-analysis, this demonstrates the real possibility of EVLP to reduce the number of lung transplant wait-list deaths. Additionally, it adds weight to the argument that satisfactory results can be obtained with more than one EVLP protocol (22).
This meta-analysis is potentially limited by it being largely comprised of institutional series, however, RCT in this area are difficult since transplanting initially rejected or marginal (beyond extended criteria) donor lungs as a control arm would be ethically difficult to justify (21). Although statistical heterogeneity was low in meta-analysis endpoints, it remained moderate to high for some baseline and intraoperative characteristics. This heterogeneity is likely due to differences in centers’ donor criteria and operative methods (for example, single versus double lung), and sensitivity analysis did not demonstrate an effect on significance of outcomes. This does not however, mean that all sources of heterogeneity were able to be accounted for and the authors acknowledge that even where I2 point values were low, I2 confidence intervals often still included at least moderate heterogeneity values (16). Additionally, many studies were retrospective and included a range of recruitment years and recruitment period lengths. As EVLP methodology and knowledge (and lung transplant more broadly) is a rapidly-evolving field, this may have introduced some learning-curve type confounding effect (25).
Conclusions
Aggregated patient survival data analysis of EVLP and standard/cold-storage lung transplant recipients demonstrated no significant difference in survival at mid- to long-term follow-up. Meta-analysis demonstrated lungs accepted for EVLP had significantly lower PaO2/FiO2 ratio and a greater incidence of radiographic abnormality, however, this did not translate to a significant difference in overall survival, 30-day mortality, or primary graft dysfunction grade 3 at 72 h between EVLP and standard cohorts. EVLP offers the ability to expand the lung donor pool with acceptable mid- to long-term survival outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Andreasson AS, Dark JH, Fisher AJ. Ex vivo lung perfusion in clinical lung transplantation-State of the art. Eur J Cardiothorac Surg 2014;46:779-88. [Crossref] [PubMed]
- McMeekin N, Chrysos AE, Vale L, et al. Incorporating ex-vivo lung perfusion into the UK adult lung transplant service: an economic evaluation and decision analytic model. BMC Health Serv Res 2019;19:326. [Crossref] [PubMed]
- Sanchez PG, Bittle GJ, Burdorf L, et al. State of Art: Clinical ex vivo lung perfusion: Rationale, current status, and future directions. J Heart Lung Transplant 2012;31:339-48. [Crossref] [PubMed]
- Ghaidan H, Fakhro M, Andreasson J, et al. Ten year follow-up of lung transplantations using initially rejected donor lungs after reconditioning using ex vivo lung perfusion. J Cardiothorac Surg 2019;14:125. [Crossref] [PubMed]
- Koch A, Pizanis N, Olbertz C, et al. One-year experience with ex vivo lung perfusion: Preliminary results from a single center. Int J Artif Organs 2018;41:460-6. [Crossref] [PubMed]
- Cypel M, Keshavjee S. The clinical potential of ex vivo lung perfusion. Expert Rev Respir Med 2012;6:27-35. [Crossref] [PubMed]
- Loor G. EVLP: Ready for Prime Time? Semin Thorac Cardiovasc Surg 2019;31:1-6. [Crossref] [PubMed]
- Wallinder A, Riise GC, Ricksten SE, et al. Transplantation after ex vivo lung perfusion: A midterm follow-up. J Heart Lung Transplant 2016;35:1303-10. [Crossref] [PubMed]
- Somers J, Ruttens D, Verleden SE, et al. A decade of extended-criteria lung donors in a single center: was it justified? Transpl Int 2015;28:170-9. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Fu R, Vandermeer BW, Shamliyan TA, et al. Handling continuous outcomes in quantitative synthesis. Methods Guide for Effectiveness and Comparative Effectiveness Reviews: Agency for Healthcare Research and Quality (US); 2013.
- Higgins JP, Green S. editors. Cochrane handbook for systematic reviews of interventions. Hoboken, New Jersey: John Wiley & Sons; 2011.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Saluja R, Cheng S, Delos Santos KA, et al. Estimating hazard ratios from published Kaplan-Meier survival curves: A methods validation study. Res Synth Methods 2019;10:465-75. [Crossref] [PubMed]
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007;335:914-6. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Fildes JE, Archer LD, Blaikley J, et al. Clinical Outcome of Patients Transplanted with Marginal Donor Lungs via Ex Vivo Lung Perfusion Compared to Standard Lung Transplantation. Transplantation 2015;99:1078-83. [Crossref] [PubMed]
- Fisher A, Andreasson A, Chrysos A, et al. An observational study of Donor Ex vivo lung perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1-276. [Crossref] [PubMed]
- Sage E, Mussot S, Trebbia G, et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: The French experience. Eur J Cardiothorac Surg 2014;46:794-9. [Crossref] [PubMed]
- Nilsson T, Wallinder A, Henriksen I, et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: The French experience. Eur J Cardiothorac Surg 2019;55:766-72. [Crossref] [PubMed]
- Tikkanen JM, Cypel M, Machuca TN, et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J Heart Lung Transplant 2015;34:547-56. [Crossref] [PubMed]
- Valenza F, Rosso L, Coppola S, et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int 2014;27:553-61. [Crossref] [PubMed]
- Zeriouh M, Sabashnikov A, Mohite PN, et al. Utilization of the organ care system for bilateral lung transplantation: Preliminary results of a comparative study. Interact Cardiovasc Thorac Surg 2016;23:351-7. [Crossref] [PubMed]
- Zhang ZL, van Suylen V, van Zanden JE, et al. First experience with ex vivo lung perfusion for initially discarded donor lungs in the Netherlands: a single-centre study. Eur J Cardiothorac Surg 2019;55:920-6. [Crossref] [PubMed]
- Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357-67. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Synthesising results when meta-analysis does not make sense. EPOC Resources for review authors, 2017. Available online: (accessed 12 December 2019).epoc.cochrane.org/resources/epoc-resources-review-authors
- Kotecha S, Hobson J, Fuller J, et al. Continued Successful Evolution of Extended Criteria Donor Lungs for Transplantation. Ann Thorac Surg 2017;104:1702-9. [Crossref] [PubMed]
- Meers C, Van Raemdonck D, Verleden GM, et al. The number of lung transplants can be safely doubled using extended criteria donors; A single-center review. Transpl Int 2010;23:628-35. [Crossref] [PubMed]