Sutureless and rapid deployment valves: implantation technique from A to Z—the Perceval valve
Introduction
Generally speaking, the implantation of Sutureless and Rapid Deployment (SURD) valves is an easy, reproducible and safe procedure. Devised to facilitate safe and effective implantation of biological aortic valve prostheses in a rapid fashion using modern deployment techniques, their use provides an almost curative treatment for patients with intermediate-to-high surgical risk and are thus suitable to fill the gap between transcatheter aortic valve implantation (TAVI) and traditional AVR (1,2). SURD valves provide excellent hemodynamic results together with reduced morbidity, resulting in shortened hospital stay and even cost reduction depending on the local reimbursement system (3).
However, these valves require specific preparatory steps prior to implantation, such as washing procedures, assembling, collapsing or crimping into delivery systems. Therefore, proper training of all surgical team members is deemed mandatory by the manufacturers (4,5). All users, regardless of their experience, should also take into account that a short learning curve is required.
These valves require surgical access to the aortic root with resection of the aortic valve and decalcification, extracorporeal circulation, as well as an aortic cross-clamping. They have specific indications and recommendations in terms of sizing and height/type of aortotomy. For these reasons, the expert recommendation is for proper education and proctoring by experienced surgeons to avoid complications.
In over ten years of usage, several recommendations have been determined to help planning the implantation of a SURD valve, based on specific surgical aspects to be addressed, as compared to the standard stented valves implantation. Let’s now take a dive into the implantation technique of the Perceval device.
Perceval sutureless valve (LivaNova)
The Perceval sutureless valve features a biological component of bovine pericardium, treated to reduce risk of calcification, and a self-expanding and elastic Nitinol alloy stent, covered by a thin coating of Carbofilm™ to improve biocompatibility. The stent consists of two rings, as well as nine connecting struts, designed to support the valve and hold it in place, with no need for any permanent sutures. The stent design mimics the anatomy of the aorta and as a result of its flexibility, follows the aortic movements to relieve stress from the leaflets.
Cannulation
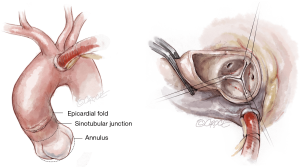
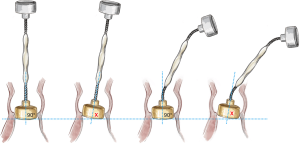
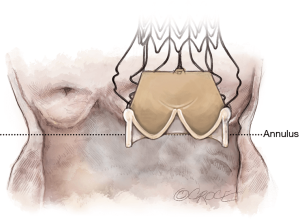
Cannulation depends on surgeon preference. Since a slightly higher aortotomy is necessary for Perceval, as compared to standard valves, a too-low aortic cannulation would not provide sufficient space, unnecessarily complicating implantation. It is recommended to perform the aortic cannulation in the distal portion of the ascending aorta or preferably, in the proximal arch, 2 cm more distally than usual, leaving 2–3 cm between cross-clamp and the aortotomy (Figure 1).
Aortotomy
The Perceval sutureless valve requires a transverse aortotomy, 1–2 cm above the sinotubular junction (STJ), slightly higher than in traditional AVR techniques. The reason is that the Nitinol stent is longer than in a traditional stented valve. If the aortotomy is too low, the stent outflow portion could interfere with the closure of the aortotomy, or may be caught within the sutures.
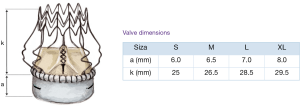
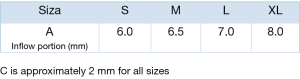
Usually, a transverse aortotomy is considered optimal if located at least 3.5 cm above the aortic annulus or at least 1cm above the STJ. Considering the XL size valve with outflow stent height of almost 30 mm (K length Figure 2), a good reference point is to perform the incision at the level of the fatty epicardial fold (plica transversa aortae).
Aortotomy extension depends on surgeon preference; it is not recommended to overextend the incision. Considering that the collapsed valve requires limited room, especially in a minimally invasive approach, a 4–5 cm aortotomy is sufficient to introduce the XL size valve.
If, for any reason, a conversion to conventional stented valve is necessary, it is possible to extend the aortotomy downwards towards the left/non-coronary (NC) commissure or to the NC sinus, according to the surgeon’s preference (hockey stick incision).
Exposure
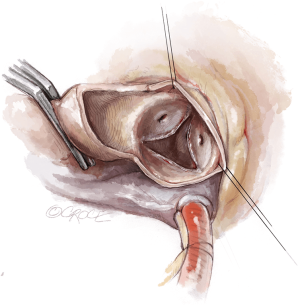
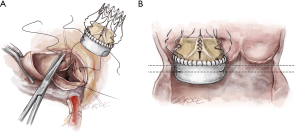
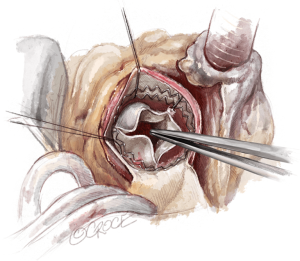
To improve exposure of the valve, most surgeons apply traction sutures on the aortic wall and to the three valve commissures (Figure 3). The use of traction sutures placed at the commissure level is not mandatory in Perceval implantation, however, their use may assist visualization and facilitate positioning of the guiding sutures, as these allow the surgeon to:
- Keep the aortotomy open;
- Bring the whole annular plane upward, making access to the valve easier;
- Allow better access to the valve during valve excision and decalcification in patients with a pliable aorta, as the tissue will fold outwards.
Valvectomy and decalcification
Leaflet excision and annulus decalcification differs minimally from the traditional AVR technique. Careful decalcification of the aortic root is recommended to avoid paravalvular leaks (PVL). This is most important around the annulus to ensure the aortic tissue is pliable and without excessive calcific irregularity, which may prevent sealing of the pericardial cloth to the annulus.
Thorough decalcification remains a crucial aspect, given the importance of ensuring full radial expansion of the proximal crown, which represents the first step of a correctly-performed valve deployment. Eccentric/bulky protruding intraluminal calcification must be removed to avoid impairment of strut expansion. Inadequate decalcification or residual calcium may cause a non-geometrical expansion or an uneven surface, which can lead either to PVL or an asymmetric intra-prosthetic regurgitation.
However, extensive decalcification is not required and care must be taken to avoid creation of annular defects that would preclude the use of a Perceval valve.
Bulky calcium deposits in the left ventricular outflow tract (LVOT) below the annulus may prevent optimal expansion of the inflow portion of the stent, particularly if this is in the NC cusp (NCC), extending to the anterior mitral leaflet. These deposits should be removed during decalcification to avoid PVL and/or incorrect valve positioning.
Warning
Ensure that the aorto-mitral curtain remains intact. If it is injured, the Perceval valve will not be able to cover this leak and prevent bleeding into the anterior mitral leaflet. In such cases, repair the injury with single stiches of 4-0 or 5-0 Polypropylene suture; single sutures instead of running sutures will avoid narrowing of the annulus.
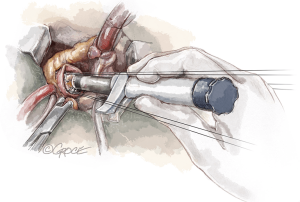
Decalcification is critical in areas close to the commissures. Here, the calcium deposits could impair commissural strut expansion (Figure 4).
Severe LVOT hypertrophy may prevent optimal expansion of the inflow portion of the stent. If present, myectomy is recommended. It is very important to keep in mind the dimension of inflow portion that protrudes into the LVOT (Figure 5).
Sizing
Perceval has a larger diameter than the indicated annuli range in order to apply a controlled expansion force for valve sealing and anchoring once implanted. Therefore, leaflets are not designed to coapt before implantation.
Perceval is an expandable prosthesis, meaning the internal diameter is unique as it adapts in size to the patient’s true annular size (vs. conventional surgical valves which are fixed in diameter). Therefore, the surgical sizing strategy will differ between Perceval and conventional valves.
It is common practice with traditional valves to try and implant the largest size possible, to achieve better hemodynamics, as well as to refer to nomogram charts to avoid Patient Prosthesis Mismatch (PPM). This does NOT apply to Perceval. Correct sizing is crucial to the procedure and to proper functioning of the valve. Undersizing of the prosthesis may lead to central or paravalvular leakage, while oversizing may lead to elevated pressure gradients or valve malfunction.
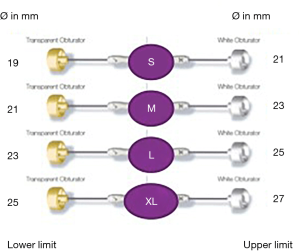
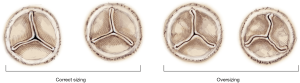
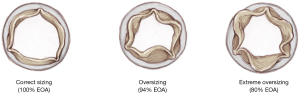
As shown in Figure 6, Perceval is designed with built-in oversizing intended to guarantee anchoring, sealing and optimal valve performance. The white barrel (representing the upper limit for a given size) is in fact smaller than the diameter of the valve out of the jar.
Each sizer represents the lower and upper limits of the Perceval valve’s respective size. The appropriate size of the prosthesis is established when the transparent obturator passes easily through the aortic annulus into the left ventricle (LV), and the white obturator remains stable above the aortic annulus (Figure 7).
It is recommended to always start the sizing with the “S” sizer, and then proceed with the sizing technique. This allows the operator to identify when resistance to the white barrel starts to increase, making identification of the correct size easier.
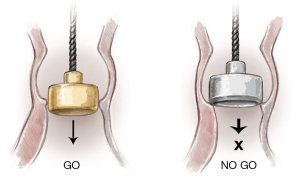
If no resistance is felt when passing either end of the sizer through the annulus, the operator should move to the next size. As soon as resistance is felt with firm pressure to the white obturator, this will indicate the correct size (Figure 8).
The operator should then ensure that the obturator is positioned perpendicularly to the annulus (Figure 9). The white obturator should neither deform the annulus during the sizing procedure, nor be forced through it with too much pressure. Therefore, in cases of doubt, first verify that the barrel of the valve sizer has entered the annulus perpendicularly to the annulus plane. Then, if the white obturator remains above the annulus or is blocked within the annulus, requiring firm traction for retrieval, choose the same size valve (not the larger size).
Note
- To confirm the correctly sized valve a De Bakey forcep will also be able to be inserted into the aortic annulus alongside the correctly sized yellow obturator.
- An oversized Perceval valve will NOT guarantee a better hemodynamic performance. On the contrary, if oversized, Perceval will likely show suboptimal expansion, which may subsequently result in higher gradients.
- In a borderline case, when the annulus is between two sizes, consider the overall dimension of the aortic root (sinuses and STJ diameter) for size choice. In this case, you can divide the STJ diameter (Ø) by 1.3 to identify the smallest implantable size (e.g., an STJ of 31 mm will provide 23.8 as minimum allowed annulus; in such cases, the smallest valve to be implanted is size L).
The effect of oversizing is well described by Cerillo et al. (6) and is well represented in Figure 10.
The role of guiding sutures
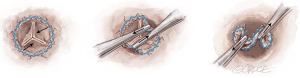
Guiding sutures must be placed to adequately hold the tension applied during the implantation of the device, as their correct placement is key to correct positioning of the valve.
The operator should position a guiding suture in each valve sinus, 2–3 mm under the leaflet hinge point, perpendicular to the annulus. The 120° distribution of the sutures can be ensured by using the sizers, as they have reference spokes which are distributed at 120°.
To avoid confusion with needles, always cut the needle corresponding to the aortic extremity of the guiding suture. This way, when you insert the guiding sutures in the eyelets, you will use the needle coming from the LVOT extremity. The needle of the LVOT extremity must be inserted in the eyelets of the collapsed valve from the inflow towards the outflow tract (Figure 12A).
LVOT extremity guidelines
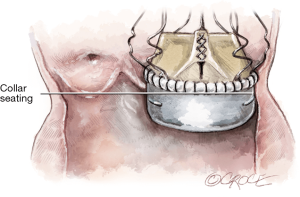
Avoid placing the guiding sutures too low in the annulus. The LVOT extremity should not be more than 2–3 mm below the annulus. The collar of the Perceval inflow should seat ABOVE the annulus.
The LVOT extremities of the guiding sutures determine the depth of the valve. Therefore, it is important that the guiding sutures are placed by inserting the needle in the LVOT (below the annulus) and exiting above the annulus. This will prevent the valve from being deployed too low or too high in the aortic root (Figure 12B).
Aorta extremity guidelines
The aortic extremity of the guiding sutures provides an important reference point prior to valve deployment. Place the guiding sutures with aortic extremity level at 2–3 mm above the annulus. This will provide a good reference point for valve positioning before the opening of the inflow ring. The rim of the collar should be at approximately the same level of the exit point of the guiding sutures.
If using commissural traction sutures, which lift the annulus plane upwards, they should be released prior to deployment of the Perceval, in order to avoid misplacement of the valve after its release.
Tension of the guiding sutures
All three guiding sutures must be pulled firmly, with similar simultaneous tension during valve deployment, without lifting the annulus plane. If tension is weak on one of the guiding sutures, the valve could tilt to the ipsilateral side. The guiding sutures should be pulled at a narrow angle close to the holder.
Before the assistant applies tension to the guiding sutures, it is important to check the proper position (height and orientation) of the collapsed valve within the annulus. Take forceps and expose the annulus, giving some side-traction on the holder, so as to expose the suture and the green eyelets. Sutures must be free from the strut of the stent. By checking each single suture, the operator can give the desired tension on the suture before passing it to the assistant. It is better if the operator does not hold the sutures, but rather keeps both hands free for deployment.
During valve deployment, ensure that the assistant is not prevented from keeping all three guiding sutures properly tensioned, and with the correct angle (Figure 13).
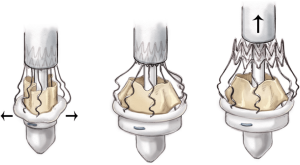
Valve deployment
Assess valve alignment from the left, right and NC sinuses. As the nadir of the NC cusp is lower, the holder should be tilted toward the surgeon to ensure appropriate seating of the valve at the annular level, and to prevent PVL.
Rotate the knob on the top of the holder clockwise (opened-lock arrow) until you hear a click, which should then produce a “tactile feeling” of the inflow ring being released. During this maneuver, make sure not to tilt the holder, which must be kept perpendicular to the annulus plane.
Take care that the sutures are not tangled around the stent posts, which could interfere with the proper seating of the device (Figure 14).
Remove the SmartClip and pull back the sliding sheath of the holder. Avoid rotational movements and keep the holder in an axial position, with respect to the aorta. Ensure that the guiding sutures are not trapped in the stent struts. The outflow of the valve will be released after withdrawal of the holder.
When valve deployment is complete, the holder should be removed from the LVOT with gentle rotational movements, paying attention not to tangle the holder in the prosthesis (Figure 15).
Inspection after deployment
A careful visual inspection is required after valve deployment to verify correct valve positioning and full expansion after the release, and to identify any possible valve folding due to oversizing.
Five points that need to be checked are:
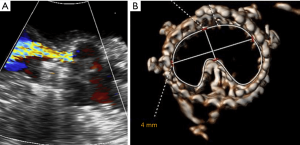
- The “Mercedes star” sign. A symmetric and linear coaptation line of the three leaflets design a typical Mercedes star.
The images in Figure 11 show the overall appearance of a deployed Perceval in different sizing conditions. A small gap is acceptable in appropriately sized valves, while the presence of a distorted leaflet coaptation line (pinwheel) oversizing should be investigated and ruled out. - The plane of coaptation of leaflets. If one of the leaflets does not coapt at the same plane as the other two, usually the corresponding portion of the collar seats below the annulus at a deeper level. For correct deployment, the Perceval collar must sit supra-annularly, while the skirt of the inflow sits intra-annularly (Figure 16).
- The annulus: the pericardial collar skirt must seat well on the top of the annulus and this is to be checked across the full 360°. It might be that in cases of higher commissures, the skirt is not covering the top of the commissures, but rather cuts the subcommissural triangle. It is important that the pericardial skirt seats well over the portion corresponding to the sinus. Mild adjustments of the skirt can also be performed if it is seating too deep. This requires pushing on the native annulus with thin De Bakey forceps while keeping a gentle tension, not pulling, on the corresponding portion of the stent.
- The LVOT should be inspected using forceps to gently open the valve. This allows additional confirmation that the inflow of the stent is covering the native anulus (Figure 17).
Therefore, if the annulus is visible from the top, the valve is placed too low. If the annulus is visible from the ventricular side, the valve is positioned too high. If a prolene guiding suture is visible, it means that the valve was deployed too high and the valve has subsequently been displaced. In this case, the Perceval has to be removed. - The strut of the stent. The inspection of how the stent is expanded and seats in the aortic root is also important. The position of the coronary ostia, the interaction of the stent with plaques or calcification of the sinuses, or the coiling of the distal outflow crown with the STJ are also vital pieces of information. An asymmetric or distorted stent can lead to malfunctioning of the valve.
Prosthesis removal
In cases of displacement or incorrect deployment, the operator can consider removal of the Perceval either for re-deployment or for converting to another prostheses. Perceval removal has been shown to be feasible and safe for the patient, and does not damage the aortic root. The recommended technique is to:
- Rinse the prosthesis with cold water as it facilitates this procedure, due to the specific features of the nitinol stent.
- Infold the valve with an “χ” shape movement, by taking the superior portion of the stent with two forceps at two opposite points and approximating them toward to the center, at the same time.
- Remove the folded valve with the two forceps (Figure 18).
Warning
Even if the valve may be collapsed safely twice prior to implantation, re-implantation after removal is an OFF-LABEL procedure, as there is no way for the manufacturer to guarantee that damage has not occurred to the Perceval valve during the removal procedure. Before re-collapsing, thoroughly check the integrity of the stent and ensure that leaflets are not damaged.
Ballooning
Balloon dilation provides optimal sealing of the valve to the aortic annulus by radially expanding the inflow stent. The balloon acts only at the inflow level (not affecting the leaflets) and does not cause any stent deformation or change to its final geometry.
Choose the balloon that corresponds to the size of the valve that is implanted. Insert the deflated balloon across the valve so that the blue marker is aligned to the free edge of the leaflets, keeping the shaft at a 90° angle to the plane of the annulus.
Inflate the balloon to 4 atm. Warm sterile saline (at 37 °C) must be poured into the aortic root, as the balloon is inflated to ensure optimal valve sealing and anchoring to the annulus. Nitinol is a thermal sensitive material and the Perceval stent is designed to work at body temperature. This step is critical, especially in cases of cold cardioplegia. At low temperatures, the stent may not fully expand, therefore leading to an underestimation of oversizing. The balloon must be kept in position for 30 seconds. During balloon dilation, the catheter must be kept absolutely steady to avoid misplacement or damage to the prosthesis. This optimizes valve sealing and anchoring. Deflate the balloon, making sure it is completely deflated before removal. If a vent is placed into the LV, remember to stop the suction before ballooning, otherwise a high negative pressure can be established in LV cavity due to occlusion of the balloon.
Removal of guiding sutures
At this point, the guiding suture can be removed before closing the aortotomy. In order to remove the guiding sutures, it is better to shorten the length by cutting one edge right above the level of the aortotomy.
The guiding sutures must not be tied as this may impair, due to the anchoring of the eyelets, the optimal valve seating by distorting or tilting the valve and this can in turn, cause central or PVLs.
Closing the aorta
Before closing the aortotomy, it is important to check the space between the distal outflow crown of the stent and the margin of the aorta. Since the level of the aortotomy will move distally with respect to the annulus after declamping, particular care should be taken not to catch the valve stent in any way while closing the aortotomy, as the valve may be dislodged into the aortic root. Therefore, make sure that every stitch is passed under visual inspection to ensure proper closure. For this reason, it is preferable to perform a single running suture rather than a two-layered closure. Anchoring of the suture in the distal crown may lead to displacement of the valve once the aorta is de-clamped.
It is better to avoid side-clamping for proximal vein graft anastomosis. For this reason, it is preferable to close aortotomy first and before de-clamping, to punch the aorta and perform the proximal coronary bypass anastomosis.
Intraoperative and post-implant imaging
Transesophageal echocardiography (TEE) is an important step for quality control in every valve procedure. An echocardiographic evaluation after Perceval implantation is highly recommended to confirm correct positioning and to verify valve functionality under beating heart conditions. It is important to check for PVL immediately after de-clamping, as PVLs are most evident while the patient is still on full cardiopulmonary bypass.
A correctly sized and well-positioned Perceval should not present a greater-than-trace leakage. If a greater-than-trace leak is detected, careful evaluation is required to assess the presence of valve displacement or valve folding. A short axis view is recommended to verify if the valve is fully expanded, especially in case of elevated gradients.
It is very rare that echocardiogram parameters change during the first days of post-operative course and for this reason, a transthoracic echocardiogram (TTE) evaluation before discharge is recommended. If high gradients are detected in combination with valve regurgitation, careful evaluation must be undertaken to assess the presence of valve folding (Figure 19). In case of doubt, additional enhanced imaging (TEE, computed tomography scan) should also be considered.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glauber M, Miceli A. Minimally invasive aortic valve replacement with sutureless valve is the appropriate treatment option for high-risk patients and the "real alternative" to transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;151:610-3. [Crossref] [PubMed]
- Glauber M, Lio A, Miceli A. The gray zone is always more dark. J Thorac Cardiovasc Surg. 2016;152:110-1. [Crossref] [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [Crossref] [PubMed]
- Livanova PLC, London (UK): HVV_LS-850-0002 Rev X03 “PERCEVAL SUTURELESS AORTIC HEART VALVE - Instructions for Use”.
- Edwards Lifesciences LLC, Irvine, CA (USA): “EDWARDS INTUITY Elite Valve System Aortic Valve - Instructions for Use”.
- Cerillo AG, Amoretti F, Mariani M, et al. Increased Gradients After Aortic Valve Replacement With the Perceval Valve: The Role of Oversizing. Ann Thorac Surg 2018;106:121-8. [Crossref] [PubMed]