Transseptal chordal replacement: early experience
Introduction
There are three fundamental techniques employed in the surgical repair of primary mitral regurgitation: (I) annuloplasty; (II) leaflet repair/resection; and (III) chordal repair/replacement. This approach, in properly selected patients and in an experienced surgeon’s hands, can result in a near 100% repair rate with superb long term durability. Surgical repair of degenerative mitral disease approaches “curability.” The effort to replicate these open surgical approaches by transcatheter means has been the subject of active investigation for several decades. The eventual goal is to replicate the surgeon’s “mitral toolbox” with a variety of catheter-based techniques that could provide equally optimal clinical results with minimal morbidity.
Approximately 60% of the isolated mitral valve surgeries performed in the United States are for patients with leaflet prolapse with elongated or ruptured chords. In a published survey from the Society of Thoracic Surgeons (STS) database of mitral valve repair techniques for patients with degenerative disease undergoing operations between 2011 and 2016, annuloplasty was utilized in 96.1% of cases, leaflet resection in 58.9%, and implantation of artificial ePTFE (expanded polytetrafluoroethylene) chords in 29.2% (mean number of chords implanted was 2.0) (1).
There are multiple technical hurdles when performing surgical chordal replacement. The main challenge is to accurately implant the correct chord length to restore the optimal zone of coaptation. This is especially challenging since chordal replacement is done on an open, non-beating heart. There have been recent advances in catheter-based chordal replacement including minimally invasive transapical approaches, but these procedures have technical challenges related to chordal-leaflet attachment, entanglement in subvalvular apparatus, choice of optimal apical access site, and complications of transapical access. Developing transcatheter, transfemoral, and transseptal approaches to mitral valve chordal replacement presents the opportunity for safer and potentially earlier treatment of patients with primary mitral regurgitation. In this manuscript, we review the current transcatheter transseptal technologies in development and discuss the various issues related to device design, efficacy, durability, and clinical trial design.
Current technologies in development
The current state of device development for transcatheter transseptal chordal replacement technologies remains at an early stage. Most devices are in preclinical development phase, and there it is very limited human experience to date.
CardioMech
The CardioMech (CardioMech, Trondheim, Norway) transcatheter transseptal mitral valve repair system is in preclinical development. Limited information is available through the company website (www.cardiomech.com) and their key design objectives as stated are: (I) a one-chord solution, (II) repositionable and retrievable anchors, (III) beating heart and live tensioning with ability to evaluate the final performance of the device before release, (IV) articulated hinge for coaxial anchor placement, (V) 24 French steerable sheath for transfemoral transseptal delivery, and (VI) limited implant footprint that will not prevent the use of future technologies if needed. The basic steps of the procedure are shown in Figure 1.

ChordArt
ChordArt (Coremedic, Biel, Switzerland) is a transcatheter mitral repair system that consists of a proximal nickel-titanium anchor for leaflet securement, a distal papillary muscle anchor, and an ePTFE chord (Figure 2). Although results with a transfemoral transseptal delivery catheter have not yet been reported, the device is undergoing evaluation using conventional open surgical exposure with promising results.

ChoRe
ChoRe is a device in preclinical development for placing artificial chords on the mitral valve structure using a transcatheter procedure. The described goal is to ultimately develop a transfemoral transseptal procedure. The procedure involves puncture of the left ventricular apex from the left ventricle to the epicardial space with placement of a pledgeted anchor on the epicardial surface. This is attached to a looped ePTFE artificial chord which is then fixed to the targeted mitral leaflet segment using a pre-constructed knot that can be tightened to the final desired length (Figure 3). A recent publication describes the use of the scaled-up prototype (twice as large as intended final device size) as tested on ex vivo bovine hearts (2).
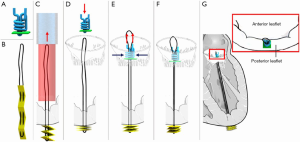
Mitral butterfly
The Mitral Butterfly (Angel Valve, Vienna, Austria) chordal mesh repair System is a unique device that attaches to the posterior leaflet and by means of a chordal mesh holds down the entire prolapsing mitral valve leaflet segment. The device consists of an atrial wing that stabilizes the entire implant in the left atrium, a swing arm that captures the polymer mesh in a central location, a polymer chordal mesh that covers the entire prolapsing segment of the mitral valve leaflet, and a clasp which fixes the implant to the leaflet (Figure 4). Evaluation of device performance in a live porcine model has been presented publicly with deployment using a 24-French transatrial delivery system under cardiopulmonary bypass (Heart Valve Society Annual Meeting, February 2020, Abu Dhabi). Delivery is currently under direct vision to the posterior leaflet of the mitral valve, and implant attachment is via a single clasp in the area in the peri-annular region. In a series of six swine, procedural success was reported as 100% with mean deployment time of eighty seconds and no device related events within ninety days follow-up.

Pipeline
The Pipeline transfemoral transseptal chordal repair system (Pipeline Medical Technologies, Inc. a wholly-owned subsidiary of W.L. Gore & Associates, Inc., Santa Rosa, California), is currently an investigational device. Femoral venous and transseptal left atrial access is gained using standard techniques. The system consists of a deflectable guide catheter, a delivery catheter, and four components which allow chordal implantation: a ventricular anchor, leaflet pledgets with GORE-TEX® sutures, a suture lock, and suture cutter. Figure 5 illustrates the steps of the procedure.
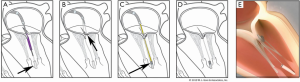
We recently reported the first human use of the Pipeline device in a 56-year-old man with symptomatic P2 prolapse (NYHA Class III) and severe MR with normal LV systolic function (3). The first step of this procedure was placement of the ventricular anchor. The target location was at the level of the base of the papillary muscles such that the top of the anchor “tower” is at the same level as the native papillary muscles. Next, the patient underwent transcatheter placement of two pledgeted sutures on the P2 prolapsed segment, and these were attached and tensioned onto a ventricular anchor under real time transesophageal (TEE) guidance with anatomic restoration of the P2 geometry and elimination of mitral regurgitation. Although the patient had an uneventful immediate post-procedure course, there was partial anchor displacement prior to discharge and the patient subsequently underwent successful surgical mitral valve correction. Learnings from this case will be applied to future device iterations with improved anchoring outcomes.
Unique procedural aspects of the Pipeline procedure are that pledgeted chordal attachment to the prolapsed leaflet segment is done from the left atrial side, avoiding entanglement that can occur with the transapical ventricular approach. Also, the ventricular anchor has a unique “neopapillary tower” design which elevates the chordal attachment point to a level similar to the native papillary muscles. This has several advantages including shorter and more physiologic chordal lengths that mimic the native chordal length. Shorter chords may also result in lower chordal stress and less chance of chordal failure over time as can be seen with longer transcatheter chords. Finally, since the chordal attachment point is at the same level as the native papillary muscles, there is less need to locate the optimal anchoring point to achieve optimal alignment, as is required with the current transapical chordal replacement devices (4).
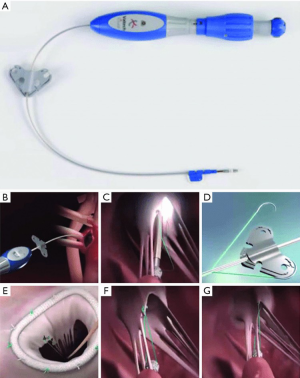
Valtech V-Chordal
The V-Chordal adjustable artificial chordal system (Valtech, Or Yehuda, Israel) is a surgical transcatheter technology which allows on-pump chordal implantation with off-pump beating heart length adjustment. The procedure is accomplished via a left transatrial approach, crossing the mitral valve and placing a helical anchor at the head of the intended papillary muscle. The helical anchor has a unique tensioning coil which can be adjusted after implantation to alter the final chordal length. An ePTFE chordal loop is pre-attached to this anchor and sutured to the mitral leaflet. After weaning from cardiopulmonary bypass, final chordal length adjustment can be performed on a beating heart under TEE guidance (Figure 6). Published preclinical feasibility has been reported in an ovine model, but there have not been further reports of a transfemoral system under development (5).
Discussion
Transfemoral transseptal chordal replacement is a promising transcatheter technique for treating patients with symptomatic mitral regurgitation due to leaflet prolapse/flail. The feasibility and efficacy of catheter-based chordal implantation has been demonstrated using the transapical approach. Although fully transseptal systems are less invasive and have numerous potential advantages, there remains much unknown about these new techniques. A summary of pros and cons for transseptal chordal replacement systems is summarized in Table 1.
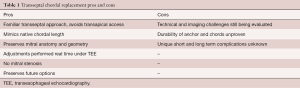
Full table
Advantages to a transseptal approach include the fact that it is a familiar approach to many users who have become adept at transcatheter edge-to-edge repair systems, such as MitraClip (Abbott, Santa Clara, California). A transseptal approach is less invasive then the transapical approach and would avoid certain complications such as apical injury, pleural or pericardial effusions, and the need to select the optimal apical access site (typically not the true apex) for the desired chordal trajectory (6). As described above, transseptal systems can mimic the native chordal length, restoring the natural geometry and potentially reducing chordal stress seen with longer chords (7). The ability to place several chords to a prolapsed segment and then adjust them real-time under TEE guidance is also an advantage over open surgical chordal replacement, since fine adjustments can be made on the beating heart for optimizing the zone of coaptation. Other advantages of chordal replacement in general are preservation of normal leaflet motion, avoiding mitral stenosis, and leaving the valve open to future options such as subsequent chordal replacement, annuloplasty, edge-to-edge repair, or transcatheter mitral valve replacement.
There are numerous technical challenges to a transseptal approach. The integrity of the ventricular anchor is likely to be a challenge with these new technologies since the left ventricular wall is highly variable in terms of thickness and in the degree of trabeculations present. Ventricular anchors will need to be robust and not prone to failure, since loss of anchoring will result in a loss of efficacy and may require surgical intervention for device removal. Optimal patient selection also remains to be fully elucidated. There has been extensive experience with the Neochord DS 1000 device (Neochord Inc., St. Louis Park, Minnesota), and the concept of the leaflet-to-annulus index (LAI) has been proposed as a predictor to determine which patients would respond best to chordal replacement. In one published study by Colli et al., the authors concluded that the ratio of the sum of the anterior and posterior leaflet lengths divided by the antero-posterior (AP) mitral annular diameter should be ≥1.25 to allow for adequate excess leaflet length and adequate zone of coaptation. Patients with a baseline LAI ≥1.25 were more likely to have ≤ mild MR at 1 year follow-up (8).
Ultimately, these technologies will need to be compared in a randomized trial against conventional surgical repair. The initial target population for these devices will be straightforward central segment prolapse premises (P2 or A2 prolapse/flail), and often these patients are younger and have lower surgical risk. Transseptal outcomes will need to be predictable and highly efficacious to compete against surgical mitral valve repair in experienced hands. For patients at high or prohibitive risk for surgical mitral valve repair, transseptal chordal replacement will need to be compared against other transcatheter approaches currently available, such as MitraClip. It is also intriguing to speculate that should transseptal chordal placement prove safe and efficacious, earlier treatment of patients with prolapse may be considered to alter the natural history of these patients: specifically, avoiding the subsequent development of heart failure, atrial fibrillation, or other adverse cardiac sequelae.
Summary
Transseptal chordal replacement is a promising emerging technique for the correction of degenerative mitral regurgitation. Multiple systems are in development each using different ventricular anchoring and leaflet-chordal attachment techniques. This technology may allow treatment of patients with minimal morbidity, while preserving native valve geometry and future transcatheter options, and perhaps ultimately allowing treatment of patients earlier in the disease process.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: SFB and JHR consultants to Abbott, Boston Scientific, and Pipeline Medical Technologies, a wholly-owned subsidiary of W. L. Gore & Associates. Inc.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gammie JS, Chikwe J, Badhwar V, et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg 2018;106:716-27. [Crossref] [PubMed]
- Culmone C, Ali A, Scali M, et al. ChoRe: A device for trans-catheter chordae tendineae repair. Proc Inst Mech Eng H 2019;233:712-22. [Crossref] [PubMed]
- Rogers JH, Ebner AA, Boyd WD, et al. First-in-Human Transfemoral Transseptal Mitral Valve Chordal Repair. JACC Cardiovasc Interv 2020;13:1383-5. [Crossref] [PubMed]
- Colli A, Bizzotto E, Manzan E, et al. Patient-Specific Ventricular Access Site Selection for the NeoChord Mitral Valve Repair Procedure. Ann Thorac Surg 2017;104:e199-e202. [Crossref] [PubMed]
- Maisano F, Cioni M, Seeburger J, et al. Beating-heart implantation of adjustable length mitral valve chordae: acute and chronic experience in an animal model. Eur J Cardiothorac Surg 2011;40:840-7. [Crossref] [PubMed]
- Colli A, Manzan E, Aidietis A, et al. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation. Eur J Cardiothorac Surg 2018;54:460-6. [Crossref] [PubMed]
- Grinberg D, Cottinet PJ, Thivolet S, et al. Measuring chordae tension during transapical neochordae implantation: Toward understanding objective consequences of mitral valve repair. J Thorac Cardiovasc Surg 2019;158:746-55. [Crossref] [PubMed]
- Colli A, Besola L, Montagner M, et al. Prognostic impact of leaflet-to-annulus index in patients treated with transapical off-pump echo-guided mitral valve repair with NeoChord implantation. Int J Cardiol 2018;257:235-7. [Crossref] [PubMed]






