Long-term outcomes of etiology specific annuloplasty ring repair of ischemic mitral regurgitation
Introduction
Contemporary surgical treatment of ischemic mitral regurgitation (IMR) with undersized complete ring annuloplasty has yielded suboptimal clinical results with high recurrence rates at 2 years and no mortality benefit over valvular replacement (1,2). However, durable repair confers improved left ventricular (LV) remodeling (1). IMR recurrence is thought to arise from altered subvalvular geometry (3) and persistent leaflet tethering (4), which is not addressed with conventional reductive annuloplasty. Etiology-specific annuloplasty prostheses have been developed to explicitly target the annular and ventricular geometric perturbation associated with IMR (5,6) in hopes of providing more durable results in addressing the “ventricular” etiology (7) of mitral insufficiency. The GeoForm annuloplasty ring (Edwards Lifesciences, Irvine, CA, USA) offers aggressive annular septal-lateral reduction (8) and three-dimensional design to simultaneously enhance leaflet coaptation and alter subvalvular geometry (9) in patients with IMR. We (10) and other investigators (11,12) have demonstrated encouraging mid-term clinical performance of this prosthesis in IMR patients, but long-term data have thus far been lacking. In the current report, we review the long-term clinical outcomes of all patients who underwent repair of IMR with a GeoForm ring at our institution over a 10-year period.
Methods
The Institutional Review Board approved our study on March 16, 2010 (SH IRB #2010-034), which was in full compliance with its policies and procedures. Patient consent was waived.
Study design
We performed a retrospective review of the data from all patients entered in our local Society of Thoracic Surgeons database from January 2005 to May 2015 who had undergone implantation of the GeoForm annuloplasty ring for treatment of IMR. The general indication for clinical use of the GeoForm prosthesis in our practice was significant (at least moderate) functional or IMR with low ejection fraction (<35%) and a significantly dilated mitral annulus and LV cavity. All patients were included, regardless of the pre-operative degree of mitral insufficiency and additional or previous cardiac procedures.
Surgical procedure
The operative procedure was performed as described previously (10). The heart was typically arrested with cold anterograde blood cardioplegia, and subsequently, cold retrograde blood cardioplegia was re-delivered every 15 to 20 minutes throughout the procedure. A septal temperature probe was used, and a temperature <10 °C was achieved with each dose. Before cross-clamp removal, 500 mL of warm retrograde cardioplegia was delivered, followed by 5 minutes of warm retrograde blood perfusion as a “hot shot”. The implanted rings were sized using the clinical criteria of intertrigonal distance and subsequent downsizing of one to two sizes, depending on the degree of LV dysfunction, patient size, and LV chamber remodeling. The ring was implanted using multiple [15–20] 2-0 Ethibond sutures to better distribute the tension and reduce the risk of prosthesis dehiscence. The anterior portion of the ring was tied down first. Subsequently, alternating sutures were tied, starting from each trigone and working toward the center of the posterior annulus. The mid-posterior “hump” of the ring was tied last to minimize the chance of prosthesis dehiscence from high suture tension.
Data collection and analysis
The perioperative mortality and 30-day events were queried directly from The Society of Thoracic Surgeons database. Follow-up regarding late mortality was conducted using the Michigan Social Security Death Index and our electronic medical records and was 100% complete. The last follow-up was in December 2018. The pre- and post-operative echocardiograms were reviewed to assess the degree of MR, ventricular function and geometry pre-operatively, and at the last available study at least 1 month beyond surgery. The degree of MR was determined by visual inspection of the extent of the regurgitant jet traversing the left atrium, reversal of flow in the pulmonary veins, and measurements of the vena contracta width. The MR was graded using these standard clinical criteria on a 0 to 4+ scale. The pre-operative patient characteristics and post-operative morbidity was defined using the Society of Thoracic Surgeons criteria.
Statistical analysis
Normally distributed and continuous variables are reported as mean ± standard deviation, non-normal data as median (interquartile range), and categorical variables as count (percent). The Kaplan-Meier method was used to determine actuarial survival and freedom from recurrent moderate or severe MR. All descriptive statistics and graphics were generated using SAS (SAS Enterprise Guide software, Version 7.1, SAS Institute Inc., Cary, NC, USA).
Results
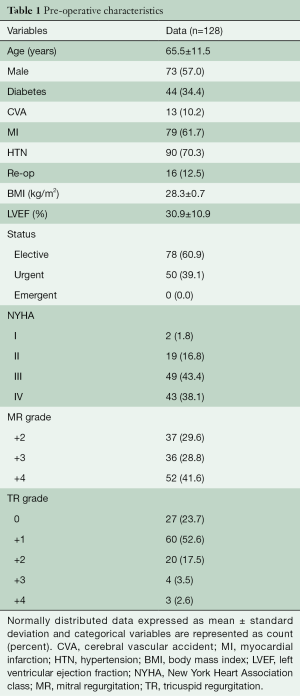
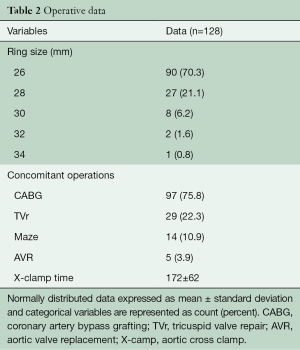
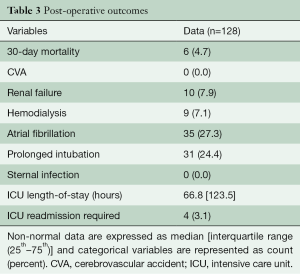
A total of 128 consecutive patients underwent implantation of the GeoForm annuloplasty ring for the treatment of IMR during the study period. The pre-operative characteristics of the study patients are shown in Table 1. This data demonstrates that our patients had severe LV dysfunction and significant symptomatology, with 81.5% being either in New York Heart Association (NYHA) class III or IV heart failure pre-operatively. Over 90% of patients were implanted with a size 26- or 28-mm ring (Table 2), with a mean ring size of 26.8±1.5 mm. Concomitant procedures, most commonly CABG, were frequently performed, yielding a mean aortic cross-clamp time of almost 3 hours. Clinical outcomes at 30 days are summarized in Table 3 and reveal acceptable morbidity and mortality for this high-risk patient cohort.
Full table
Full table
Full table
Long-term outcomes
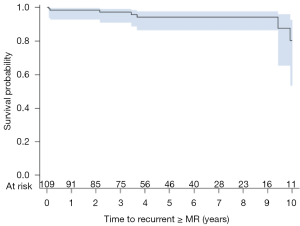
Of patients surviving the peri-operative period, 89% (109/122) had follow-up echocardiography beyond 1 month with a mean follow-up of 59±39 months. Total echocardiographic follow-up was 535 patient-years. LVEF improved from 30.2%±9.7% to 37.7%±14.1% (P<0.001) while end-diastolic (5.92±0.93 to 5.32±0.92 cm, P<0.001) and end-systolic (4.98±0.95 to 4.38±1.11 cm, P<0.001) LV diameters decreased, as compared to pre-operative values. The mean mitral valve gradient at the last follow-up was 4.7±1.9 mmHg. Seven patients were found to have recurrent moderate or severe IMR in follow-up to 10 years (Figure 1) with three being due to ring dehiscence. One-, 5-, and 10-year freedom from recurrent moderate or greater IMR was 98%, 94%, and 80% respectively.
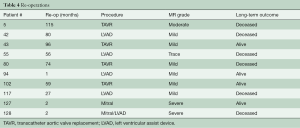
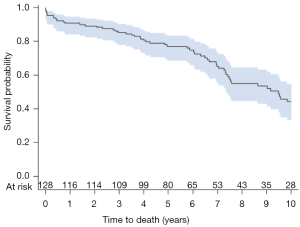
Ten patients required re-operation with the time interval from index procedure, type of intervention, and degree of MR on intra-operative trans-esophageal echocardiogram (TEE) summarized in Table 4. Three patients had recurrent MR due to ring dehiscence with patient #127 operated on for re-repair with a different annuloplasty ring, and patient #128 undergoing left ventricular assist device (LVAD) implantation without mitral intervention. A third patient with ring dehiscence and moderate MR first noted at 44 months post-operatively was not re-operated on due to several co-morbidities and expired at 88 months with severe MR. Two patients with ring dehiscence had impressive pre-operative LV chamber dilation with end-diastolic diameters of 76 and 86 mm, respectively. Patient #94 required mechanical support at the index operation, which was then converted to a durable LVAD at 1 month. The device was subsequently explanted due to infection and this patient has only trace MR at last evaluation, i.e., 59 months post-LVAD removal. Long-term post-operative survival for the entire patient cohort is shown in Figure 2. One-, 5-, and 10-year survival was 91%, 77%, and 44%, respectively.
Full table
Discussion
IMR continues to frustrate clinicians due to high rates of repair failures and sub-optimal long-term survival. Etiology-specific annular prostheses were developed to improve clinical outcomes, yet long-term data is lacking. In 128 patients undergoing ischemic mitral valve repair with an etiology-specific annuloplasty ring, we found acceptable peri-operative mortality and morbidity with low rates of recurrent moderate or severe mitral insufficiency at long-term follow-up.
In a large patient cohort from the Cleveland Clinic (13), 5-year survival for comparable patients undergoing annuloplasty repair of IMR and concurrent coronary artery bypass grafting (CABG) was reported to be 74%, and is similar to our experience. Mid-term survival outcomes reported by other investigators with the GeoForm prosthesis (11,12) reflect our results and approximate the 62% 7-year survival reported with the ETlogix ring (14). At 10 years, our patient survival decreased to 44%, similar to the 10-year survival of 39% reported by the Cleveland Clinic (13) and the 48% 12-year survival reported in IMR patients repaired predominantly with the classic Carpentier-Edwards ring (15). A German study of seventy patients with IMR repaired with the GeoForm prosthesis found 71% freedom from recurrent moderate or severe MR at 4 years (12), consistent with 75% freedom from ≥2+ MR at 3.5 years reported by Alfieri’s group (11). Although there is significant heterogeneity in the rates of MR recurrence after ring repair (16), these results and the current report are a significant departure from recently published randomized data on repair versus replacement of IMR (1,2). Goldstein and colleagues (1) reported a 58.8% recurrence of moderate or severe MR at 2 years after undersized complete annuloplasty repair of severe IMR, while a recurrence rate of 11.2% at 2 years was subsequently reported for patients with only moderate IMR undergoing concurrent CABG at the time of annuloplasty repair (2). As only 41% of our patients had severe MR at the time of the operation, it is feasible that the difference in recurrence rates between our data and Goldstein’s report is partially due to baseline severity of mitral insufficiency. Furthermore, ours was a retrospective study without reference-lab evaluation of MR, and it is possible that the degree of recurrent insufficiency was underestimated. However, the 10 patients who underwent re-operation after the index procedure were found to have their pre-operative transthoracic echocardiogram (TTE) assessment of MR consistent with intra-operative TEE findings. Overall, there was significant LV reverse remodeling observed in our patients, and improvement in ejection fraction from 30%±10% to 38%±14%, indirectly suggesting good control of mitral insufficiency. Interestingly, the high 2-year recurrent MR rate reported in the Cardiothoracic Surgical Trials Network Investigators randomized trial was not associated with improvement in LV function (42%±12% vs. 43%±12% for pre- and post-operatively, respectively) (1,17), although the rate of concurrent myocardial revascularization of 73.8% was similar to our CABG rate of 75.5%. Clinical outcomes with other etiology-specific annuloplasty rings corroborate our data as Gatti (14) reported freedom from moderate or severe MR of 93.1% at 7 years in 190 patients with IMR that were repaired with the IMR ETlogix ring (Edwards Lifesciences, Irvine, CA, USA). Approximately 33% of these patients had severe mitral insufficiency pre-operatively. Similarly, Campisi (18) demonstrated freedom from recurrent ≥2+ MR of greater than 90% at 5 years in 157 consecutive patients with predominantly moderate-severe IMR repaired with the ETlogix prosthesis. This data and published GeoForm outcomes suggest improved results of etiology-specific prostheses over standard undersized annuloplasty repair of IMR, although without randomized studies or direct comparisons, the putative benefit is speculative.
We hypothesize that the etiology-specific design of the GeoForm ring was a significant contributor to the durable control of IMR observed in our study. The prosthesis was designed to increase leaflet coaptation through remarkable annular septal-lateral reduction while counteracting tethering forces by altering the three-dimensional shape of the posterior annulus (9). The inner septal-lateral dimension of a size 32 mm GeoForm ring is smaller than any 24 mm sized ring available on the market, including the IMR ETlogix prosthesis (19). To achieve a similar degree of annular septal-lateral reduction, the two-dimensional areas for the implanted rings would be 402 and 225 mm2, for GeoForm and IMR ETlogix, respectively. Avoidance of severe annular area reduction may be important for valvular competence, as a post-hoc analysis of MR recurrence from the Cardiothoracic Surgical Trials Network Investigators (4) found size-mismatch between implanted ring size and LV end-systolic diameter to be an independent predictor of recurrent insufficiency in patients with moderate and severe IMR. The size mismatch was thought to exacerbate posterior leaflet tethering, especially in dilated LV cavities with lateral papillary muscle displacement. However, these mechanisms may be mitigated by the design of the GeoForm ring, which was demonstrated to reduce the posterior leaflet tenting area in acute ovine IMR (19) and has been shown to be the only etiology-specific ring to reduce ventricular septal-lateral dimension (20). Recent computational analysis confirmed more effective leaflet coaptation with the GeoForm ring, as compared to the Physio II prosthesis (21). Clinically, three-dimensional echocardiography of patients undergoing IMR repair with the GeoForm ring has revealed alteration of annular geometry and reduction of tenting volume (22). Maintenance of orifice area with the GeoForm prosthesis is further reflected in the acceptable mean mitral gradient of 4.7 mmHg in long-term follow-up, which is comparable to prior reports (11,12) and would not be expected to negatively affect survival (23).
The unconventional geometry of the GeoForm ring is postulated to increase valvular stresses, as the annulus is made to conform to its three-dimensional shape. Mathematical modeling has confirmed these hypotheses (24). Although a high number of sutures were used to anchor the annuloplasty, we observed three mechanical device disruptions in our study, accounting for almost half of the cases with recurrent MR. Three cases of ring dehiscence were also reported by Guenzinger (12) in a series of seventy GeoForm patients, however only reported one device dehiscence in a patient with endocarditis in the study by De Bonis (11). Two device failures in our cohort were associated with significantly dilated left ventricles, suggesting that excessive stresses may be present when massive ventricles are made to conform to the exaggerated septo-lateral reduction induced by the prosthesis, and perhaps valve replacement should be considered in these scenarios.
Limitations
Several important limitations of our study need to be considered to interpret the results in the appropriate context. This was a single-center retrospective study and patient selection was at the discretion of the surgeon. As mentioned above, only 40% of patients had pre-operative severe MR, perhaps contributing to the low rate of recurrent IMR observed in our study. However, long-term rates of recurrent MR were as good as 2-year data for patients with only moderate IMR undergoing CABG and concurrent ring repair (2). The echocardiographic follow-up, although adequate, was not 100% complete, and it is feasible that patients lost to follow-up were harboring significant MR. The last available echocardiographic assessment of deceased patients was included in the study, but due to the considerable mortality rate in long-term follow-up, it is feasible that deceased patients may have had significant MR prior to expiring. The available echocardiographic studies were transthoracic and retrospective and may have underestimated recurrent insufficiency. However, of the 10 patients who underwent re-operation, the intra-operative TEE findings were consistent with pre-operative imaging, although core lab data from the STICH trial have shown only modest correlation between TTE and TEE in grading of severity of IMR (25).
In conclusion, we found the GeoForm ring to offer acceptable peri-operative mortality and morbidity, good mid-term survival, and durable long-term control of moderate or severe IMR. This data suggests that etiology-specific rings for IMR may offer better clinical outcomes than standard undersized prostheses, and this therefore warrants consideration of further studies.
Acknowledgments
Funding: Internal institutional funds were used to support this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1. [Crossref] [PubMed]
- Capoulade R, Zeng X, Overbey JR, et al. Impact of left ventricular to mitral valve ring mismatch on recurrent ischemic mitral regurgitation after ring annuloplasty. Circulation 2016;134:1247-56. [Crossref] [PubMed]
- Votta E, Maisano F, Bolling SF, et al. The GeoForm disease-specific annuloplasty system: a finite element study. Ann Thorac Surg 2007;84:92-101. [Crossref] [PubMed]
- Daimon M, Fukuda S, Adams DH, et al. Mitral valve repair with Carpentier-McCarthy-Adams IMR ETlogix annuloplasty ring for ischemic mitral regurgitation: early echocardiographic results from a multi-center study. Circulation 2006;114:I588-93. [Crossref] [PubMed]
- Bolling SF, Pagani FD, Deeb GM, et al. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 1998;115:381-6; discussion 387-8. [Crossref] [PubMed]
- Bothe W, Swanson JC, Ingels NB, et al. How much septal-lateral mitral annular reduction do you get with new ischemic/functional mitral regurgitation annuloplasty rings? J Thorac Cardiovasc Surg 2010;140:117-21. [Crossref] [PubMed]
- Votta E, Vismara R, Redaelli A, et al. Response of two annular prostheses to functional mitral regurgitation main determinants: an in vitro evaluation. ASAIO J 2010;56:491-6. [Crossref] [PubMed]
- Timek TA, Hooker RL, Collingwood R, et al. Five-year real world outcomes of GeoForm ring implantation in patients with ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2014;148:1951-6. [Crossref] [PubMed]
- De Bonis M, Taramasso M, Grimaldi A, et al. The GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathy. Eur J Cardiothorac Surg 2011;40:488-95. [Crossref] [PubMed]
- Guenzinger R, Schneider EP, Guenther T, et al. Three-dimensional valve repair-the better care? Midterm results of a saddle-shaped, rigid annuloplasty ring in patients with ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2014;148:176-82. [Crossref] [PubMed]
- Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007;49:2191-201. [Crossref] [PubMed]
- Gatti G, Dell'Angela L, Pinamonti B, et al. Asymmetric ring annuloplasty for ischemic mitral regurgitation: early and mid-term outcomes. J Heart Valve Dis 2014;23:695-706. [PubMed]
- Magne J, Girerd N, Sénéchal M, et al. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short-term and long-term survival. Circulation 2009;120:S104-11. [Crossref] [PubMed]
- Magne J, Sénéchal M, Dumesnil JG, et al. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology 2009;112:244-59. [Crossref] [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [Crossref] [PubMed]
- Campisi S, Fuzellier JF, Haber B, et al. Mid-term results of mitral valve repair for ischemic mitral regurgitation with ETlogix ring: a single-center study. Int J Cardiol 2016;222:924-30. [Crossref] [PubMed]
- Bothe W, Kvitting JP, Stephens EH, et al. Effects of different annuloplasty ring types on mitral leaflet tenting area during acute myocardial ischemia. J Thorac Cardiovasc Surg 2011;141:345-53. [Crossref] [PubMed]
- Bothe W, Kvitting JP, Rausch MK, et al. Do annuloplasty rings designed to treat ischemic/functional mitral regurgitation alter left-ventricular dimensions in the acutely ischemic ovine heart? J Thorac Cardiovasc Surg 2019;158:1058-68. [Crossref] [PubMed]
- Choi A, McPherson DD, Kim H. Computational virtual evaluation of the effect of annuloplasty ring shape. Int J Numer Method Biomed Eng 2017;33. [Crossref] [PubMed]
- Armen TA, Vandse R, Crestanello JA, et al. Mechanisms of valve competency after mitral valve annuloplasty for ischaemic mitral regurgitation using the Geoform ring: insights from three-dimensional echocardiography. Eur J Echocardiogr 2009;10:74-81. [Crossref] [PubMed]
- Rubino AS, Onorati F, Santarpia G, et al. Impact of increased transmitral gradients after undersized annuloplasty for chronic ischemic mitral regurgitation. Int J Cardiol 2012;158:71-7. [Crossref] [PubMed]
- Kong F, Pham T, Martin C, et al. Finite element analysis of annuloplasty and papillary muscle relocation on a patient-specific mitral regurgitation model. PLoS One 2018;13:e0198331. [Crossref] [PubMed]
- Grayburn PA, She L, Roberts BJ, et al. Comparison of transesophageal and transthoracic echocardiographic measurements of mechanism and severity of mitral regurgitation in ischemic cardiomyopathy (from the Surgical Treatment of Ischemic Heart Failure Trial). Am J Cardiol 2015;116:913-8. [Crossref] [PubMed]






