Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes
Introduction
The NeoChord procedure is an early-stage surgical procedure that has been approved in Europe to address degenerative mitral valve (MV) regurgitation (DMR) independently of patient’s risk profile and age (1,2). The NeoChord DS1000 Artificial Chordae Delivery System (NeoChord Inc., St. Louis Park, MN, USA) enables placement of expanded polytetrafluoroethylene chords on a beating heart through transapical access under direct two- and three-dimensional transesophageal echocardiography (TEE) guidance (3,4). Good early results have been previously presented in a large single center experience (5) and in a multicenter European study (6). Furthermore, the successful improvements made in this novel technique thanks to procedure standardization, patient selection optimization, and learning curve stabilization has been demonstrated as well (7). Early clinical and echocardiographic efficacy has also shown to be maintained up to one year confirming the excellent safety profile with stable low mortality rate. Surgical-like MV functional status was achieved in most of the cases and significant ventricular and atrial reverse remodeling and no significative periprocedural left ventricle function impairment were observed (8). Furthermore, the patient population which met the refined anatomical selection criteria (isolated posterior leaflet prolapse, or isolated anterior leaflet prolapse with adequate leaflet tissue overlap) showed better echocardiographic results (9). However, the long-term durability of the repair, since lacking the annuloplasty ring, and the impact of the procedure on long-term survival remain important clinical questions. In this report, we present the mid-term clinical follow-up results through three years of our large single center experience.
Methods
All consecutive patients that underwent MV repair with the NeoChord procedure at University of Padua between November 2013 and June 2019 were included in the current analysis. All the included patients presented with severe symptomatic DMR due to prolapse or flail of one or both mitral leaflets. The surgical indication was based on clinical and anatomical characteristics and the patient’s personal preferences regarding treatment independently from age and surgical risk profile. This study is an independent investigator-initiated extension of the NeoChord TACT post-market surveillance registry (NCT01784055), approved by the local IRB.
All patients underwent preoperative transthoracic echocardiography (TTE) and TEE to establish the grade and mechanism of mitral regurgitation (MR). The NeoChord procedure was performed as previously described (3,4). After the procedure, patients underwent clinical and echocardiographic follow-up at one, three, six, 12 months and yearly thereafter. MR severity was graded as absent or trace (0), mild (1+), moderate (2+) and severe (≥3+) according to American Society of Echocardiography criteria (10). Outcomes were defined according to the Mitral Valve Academic Research Consortium guidelines in an intention-to-treat analysis (11). Procedural success was defined as the placement of at least two neochordae and achievement of mild or less MR. The primary endpoint was patient success, a composite of procedural success (placement of at least two neochordae and mild or less MR at the end of the procedure) and freedom from death, stroke, MR > moderate, unplanned interventions related to the procedure, cardiac-related rehospitalization, or worsening New York Heart Association (NYHA) functional class at one-, two- and three-year follow-up. Patients were retrospectively categorized according to the examination of preoperative MV anatomy as follows: Type A, isolated central posterior mitral leaflet (PML) prolapse and/or flail; Type B, posterior multi-segment prolapse and/or flail; Type C, anterior or bileaflet prolapse and/or flail; Type D, paracommissural prolapse and/or flail or any type of disease with the presence of significant leaflet and/or annular calcifications.
Categorical variables were expressed as percentages and continuous variables were expressed as medians (I–III quartile, IQR). Actuarial curves were obtained by means of Kaplan-Meier analysis and statistical differences among the groups were determined by log-rank Mantel-Cox test. A P value <0.05 was considered significant. Echocardiographic comparisons were made using Student’s t-test for paired samples. SPSS statistical software was used (IBM SPSS Statistics, Version 20.0. Armonk, NY, USA).
Results
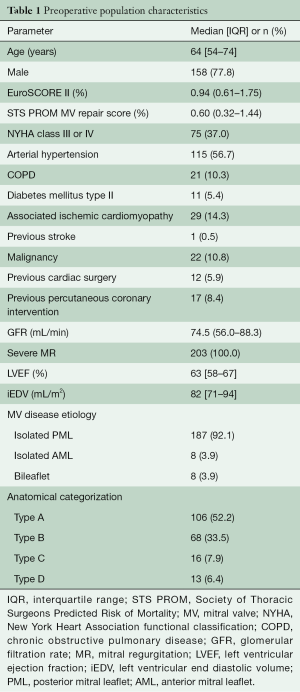
Two hundred and three patients were analyzed with a median follow-up of 24 months (IQR, 9–36 months); 50 patients (25%) had completed the three-year follow-up visit. Demographic and preoperative echocardiographic data are summarized in Table 1. Median age was 64 years (IQR, 54–74 years), median STS PROM was 0.60% (IQR, 0.32–1.44%) and EuroSCORE II was 0.94% (IQR, 0.61–1.75%). Most of the patients presented with isolated PML disease (92.1%). As a consequence, for most of the patients MV anatomy was sorted as Type A or Type B (86%).
Full table
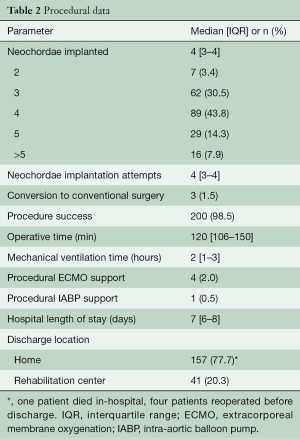
Procedural data is summarized in Table 2. Procedural success was achieved in 200 patients (99%). Three patients were converted to conventional MV repair or replacement surgery at procedure time. Of those, two patients were Type B while one presented Type D anatomy. In all cases we observed an acute chordal detachment at the leaflet end during tensioning because of an inadequate leaflet grasping obtained at deployment time. The majority of the patients (89%) received a median of three to five chords and the median operative time was 120 minutes (IQR, 106–150 minutes). Early clinical outcomes are summarized in Table 3. Two in-hospital death occurred; a 79-year-old high-risk (EuroSCORE II 8.74%) male who died following acute right ventricle dysfunction and an 87-year-old frail female for which NeoChord procedure was performed as compassionate use since she was deemed inoperable because of very high surgical risk (EuroSCORE II 5.85%) and extensive mitral annulus calcifications. Kaplan-Meier estimate of survival was 99.0%±0.7% at one and two years and 94.0%±2.9% at three years. All deaths were cardiovascular (CV), and all deceased patients were elderly with multiple severe CV comorbidities. In particular, during follow-up one patient presenting with severe symptomatic MR recurrence requiring reoperation after three years from first transapical NeoChord operation and died after surgical MV replacement because of respiratory complications and retroperitoneal hemorrhage.
Full table

Full table
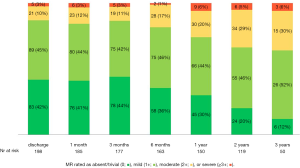
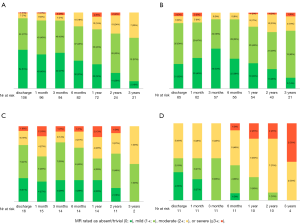
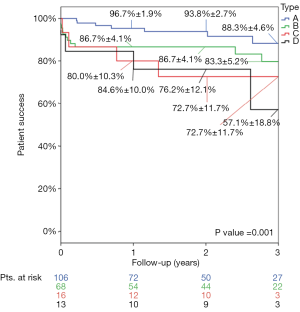
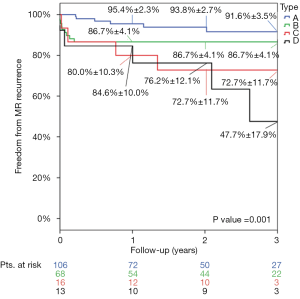
At discharge, 87% of the patients had mild or less MR (Figure 1). The percentage of patients with mild or less MR remained prevailing through three years with 86%, 74%, 66%, and 64% of the population presenting with mild or less MR at six months, one, two, and three years, respectively (Figure 2). Thirteen patients (6.4%) required reoperation for recurrent MR through three years; two Type A (1.0%), seven Type B (3.4%), three Type C (1.5%), and one Type D (0.5%). The actual rate of patients achieving patient success was 91.2%±2.0% at one year, 89.7%±2.3% at two years, and 81.2%±3.8% at three years. The rate of patient success was significantly different (P=0.001) between anatomical categorical types; higher than 88% and 83% in Type A and B patients respectively, while lower than 72% and 57% in Type C and D patients respectively (Figures 3-5). Actual freedom from more than moderate MR is depicted in Figure 4.
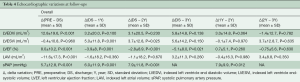
The echocardiographic analysis showed that patient’s heart chambers underwent an acute reverse remodeling corresponding to a reduction of indexed left ventricle end diastolic volume (LVEDVi), indexed left atrial volume (LAVi) and pulmonary artery pressure advocating for an effective resolution of the MR (Table 4). The indexed left ventricle end-systolic volume (LVESVi) did not change acutely but reduced overtime. The left ventricular ejection fraction (LVEF) acutely decreased as physiological response to the MR correction but significantly improved at later follow-ups. Over time, the observed overall reverse remodeling was stable thus offering a significant clinical benefit to patients.
Full table
Discussion
This report describes the largest single center experience of symptomatic DMR treatment with the NeoChord procedure. Patients with different types of prolapse or flail of one or both MV leaflets were included only on the base of pathophysiological (prolapse or flail) and anatomical criteria (Types A, B, C) (5,6). The newest echocardiographic predictors, such as the leaflet-to annulus index or the coaptation index (9), were not applied for selection. Short-term efficacy of this minimal access, minimally-invasive cardiac procedure performed without cardiopulmonary bypass, aortic cross-clamping, or cardioplegic arrest has previously been reported (12) and are conformed in this updated report. Successful placement of two or more artificial chordae was achieved in 99% of the patients resulting in acute reduction of MR severity that was maintained up to three years in the majority of the patients. Present early outcomes also validate the low rates of procedural and 30-day complications. Overall survival at three years was 94.0%±2.9%; seven patients died during follow-up with all of them affected by significant CV comorbidities. Additionally, most patients achieved patient success through three years with 63.8% of the population maintaining mild or less MR. The study also shows how the results achieved during the first period have been improved over time thanks to technique refinement, standardization of the procedural steps and patient selection criteria (7,13).
These results open up a novel interpretation of the concept long-term stability of MV repair without annuloplasty ring or band implantation. In particular, the introduction of the leaflet-to-annulus mismatch concept defined by the leaflet-to annulus index has shown to be a strong predictor of success (9) providing the most important element for patient selection. When the concept of annuloplasty was developed, leaflet prolapse was treated by leaflet resection and annular plication to reduce the size of the annulus (14) and the implant of a stabilizing annuloplasty ring aimed to reduce the risk that the area of annular plication would eventually dehisce. Nowadays in conventional open surgical MV repair based on artificial chordae implantation, annuloplasty is added as an adjunctive “parachute maneuver” to reduce leaflet strain, increase leaflet coaptation surface, and counteract unfavorable changes of the leaflet height due to ventricular dilatation over a long-term period. The durability of novel transcatheter MV repair techniques, which do not provide similar annular support, is therefore a matter of uncertainty requiring further exploration.
Our results demonstrate an excellent safety profile for the NeoChord procedure and a better than expected efficacy results for a novel technique. Survival rates were similar or better if compared to traditional surgery for the subgroup of high-risk patients. The low rate of procedural and early clinical complications is more likely to be attributed to the lack of cardiopulmonary bypass and cardioplegic arrest. In the past the transapical access has generated some concerned because of its potential damage of the LV. Interestingly, we confirmed the observation that the device insertion could generate a localized impairment of function with localized myocardial edema but because we strictly followed the patients with multiple echocardiographic examination we observed that this impairment has been reversible in all cases because of the disappearance of the trauma induced edema. The durability of the NeoChord repair, while it may not match rates seen with standard surgical repair, is comparable to other novel transcatheter technologies in the early phase of adoption. For example, results from the EVEREST II randomized trial for the MitraClip device showed similar rates of freedom from death, MV reintervention, and recurrent moderate or severe MR (15). One advantage of the NeoChord procedure compared to technologies such as the MitraClip which create a fixed coaptation point, is that it does not create any barriers to MV re-repair if necessary, making it more appealing for early-stage disease treatment in younger patients. In our study, four (30%) of the patients who required reintervention were re-repaired rather than requiring MV replacement confirming what shown by other groups (16). Moreover, two out of four were re-repaired through re-NeoChord procedure.
As technical refinements progressively enhancing the NeoChord procedure are reached, efficacy results in the treated patient population are expected to improve. It should be noted that after initiation of this study, morphological and echocardiographic patient selection criteria for suitability for the NeoChord procedure were refined in our institution (17). Therefore, some patients included in this analysis would no longer be considered suitable (e.g., paracommissural prolapse or flail, presence of calcification in the annulus or leaflets) for the NeoChord procedure after those refinements were introduced, impacting on effective results. Furthermore, minimally invasive and beating heart options, such as the NeoChord procedure, will likely encourage early referral and treatment of MV disease. Historically, patients that have been referred for MV repair are typically at a later disease stage and often present with dilated LV and consequently dilated annuli. As we have previously reported (13), such dilatation can negatively impact the durability of the NeoChord procedure through ineffective leaflet coaptation length or relative neochordae elongation resulting from significant LV remodeling. As such, the NeoChord procedure may become an attractive option for early referrals, in particular if we consider that it does not hinder a potential future repair, thus leading to better overall patient outcomes since early anatomical disease states with accompanying moderate or severe MR are those that best benefit from the procedure itself.
Additionally, recent developments in transcatheter mitral repair technologies may potentially expand the NeoChord procedure to those who were deemed unfit because of MV anatomic features. For example, transcatheter technologies that target treatment of the mitral annulus and/or mitral leaflets can be applied as a complementary therapeutic approach in a staged or sequenced manner along with the NeoChord procedure to expand beating heart treatment options for patients who present leaflet-to-annulus mismatch caused by excessive annular dilatation (18,19). Another interesting concept recently shown is the use of the NeoChord device as a flexible platform to perform chordal-based edge-to-edge repair or leaflet augmentation combining chords and pericardial patch as in traditional complex open-heart surgery (20,21). Currently, we estimate by default that approximately 15% to 20% of patients with diagnosed degenerative MV disease meet the anatomical criteria for the NeoChord procedure. However, using a combination of transcatheter therapies, it may be possible to successfully treat patients who currently due not meet criteria, for example, insufficient leaflet overlap, and/or improve the durability of the repair. Further exploration of combination transcatheter therapies for MV repair is an exciting prospect for future research and offers the opportunity to greatly increase the number of patients that may benefit from a truly minimally invasive, beating heart MV repair.
Limitations
Although this study represents the largest single-center experience reported to date, only 25% of the treated patients reached the three-year follow-up visit. Additionally, the observational design of this study without a control group is a classic limitation of surgical studies. Larger number of patients, with longer clinical and echocardiographic follow-up are required to validate the definitive value of this therapeutic approach. A randomized trial would be the best strategy, however at this stage it would not generate the most reliable results as it would mean comparing a procedure with 1,500 cases performed in five years so far with a surgical treatment that has been performed for over 40 years with 1,000 times more performed cases. Moreover, the two procedures differ in the numbers of elements of the MV that can be fixed at the same time. While the NeoChord procedure can only act on the leaflets with the implant of artificial chordae, surgical treatment always acts both on leaflets and annulus combining chordal implantation ring annuloplasty and if necessary other adjunctive techniques such as cleft closure. So said, would it be ethical to limit the surgical act only to chordal implantation wittingly overlooking ring fixation as the standard practice recommends? In other words, would it be fair to compare a procedure that always puts a “life-vest” on the valve even if not necessary, with a procedure that can only count on chordal implantation? Since surgical treatment can potentially address any possible scenario, NeoChord procedure would be disadvantaged since the beginning and it is clear that with this background any potential trial would require a very rigorous methodology for preoperative case selection, procedural success definition and outcomes evaluation in order to make the two techniques as comparable as possible.
Conclusions
This study demonstrates that mid-term safety and clinical benefit NeoChord procedure are sustained up to three years follow-up as measured by mortality, rate of re-intervention, and recurrent MR severity. Because of the low complication rate and high surgical success rate for a new transcatheter procedure, we believe that the NeoChord procedure furthered the new era of off-pump and less invasive MV interventions. The NeoChord procedure showed an encouraging progression in technique results for posterior MV leaflet disease treatment but concerns remain regarding the less optimal anatomies as Types C and D. In the future, combining the NeoChord procedure with a percutaneous annuloplasty in order to increase the coaptation length would be a possible option to improve results replicating conventional surgical MV repair and to meet the needs of those patients who would be otherwise excluded because considered unsuitable. Future detailed echocardiographic studies with larger series of patients and longer follow-up that are already ongoing will likely lead to more precise identification of the anatomic indications and will give confirm past and present studies already showing the promising results of the NeoChord procedure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: GG, AC received travel grants from NeoChord Inc. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol 2014;63:914-9. [Crossref] [PubMed]
- Kiefer P, Meier S, Noack T, et al. Good 5-Year Durability of Transapical Beating Heart Off-Pump Mitral Valve Repair with Neochordae. Ann Thorac Surg 2018;106:440-5. [Crossref] [PubMed]
- Colli A, Adams D, Fiocco A, et al. Transapical NeoChord mitral valve repair. Ann Cardiothorac Surg 2018;7:812-20. [Crossref] [PubMed]
- Colli A, Zucchetta F, Torregrossa G, et al. Transapical off-pump mitral valve repair with Neochord Implantation (TOP-MINI): step-by-step guide. Ann Cardiothorac Surg 2015;4:295-7. [PubMed]
- Colli A, Manzan E, Zucchetta F, et al. Transapical off-pump mitral valve repair with Neochord implantation: Early clinical results. Int J Cardiol. 2016;204:23-8. [Crossref] [PubMed]
- Colli A, Manzan E, Aidietis A, et al. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation. Eur J Cardiothorac Surg 2018;54:460-6. [Crossref] [PubMed]
- Colli A, Bagozzi L, Banchelli F, et al. Learning curve analysis of transapical NeoChord mitral valve repair. Eur J Cardiothorac Surg 2018;54:273-80. [Crossref] [PubMed]
- Colli A, Besola L, Montagner M, et al. Acute intraoperative echocardiographic changes after transapical off-pump mitral valve repair with NeoChord implantation. Int J Cardiol. 2018;257:230-4. [Crossref] [PubMed]
- Colli A, Besola L, Montagner M, et al. Prognostic impact of leaflet-to-annulus index in patients treated with transapical off-pump echo-guided mitral valve repair with NeoChord implantation. Int J Cardiol 2018;257:235-7. [Crossref] [PubMed]
- Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2019;32:431-75. [Crossref] [PubMed]
- Stone GW, Adams DH, Abraham WT, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015;66:308-21. [Crossref] [PubMed]
- Colli A, Manzan E, Besola L, et al. One-Year Outcomes After Transapical Echocardiography-Guided Mitral Valve Repair. Circulation 2018;138:843-5. [Crossref] [PubMed]
- Colli A, Besola L, Bizzotto E, et al. Mechanisms of recurrent regurgitation after transapical off-pump mitral valve repair with neochord implantation†. Eur J Cardiothorac Surg 2019;56:479-87. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Wróbel K, Kurnicka K, Zygier M, et al. Transapical beating heart mitral valve repair with the NeoChord system: early outcomes of a single-center experience. Wideochir Inne Tech Maloinwazyjne 2019;14:320-5. [Crossref] [PubMed]
- Manzan E, Azzolina D, Gregori D, et al. Combining echocardiographic and anatomic variables to predict outcomes of mitral valve repair with the NeoChord procedure. Ann Cardiothorac Surg 2021;10:122-30.
- von Bardeleben RS, Colli A, Schulz E, et al. First in human transcatheter COMBO mitral valve repair with direct ring annuloplasty and neochord leaflet implantation to treat degenerative mitral regurgitation: feasibility of the simultaneous toolbox concept guided by 3D echo and computed tomography fusion imaging. Eur Heart J 2018;39:1314-5. [Crossref] [PubMed]
- Colli A, Beiras-Fernández A, Ruf T, et al. Transcatheter mitral valve repair: Single stage combo approach. Rev Esp Cardiol (Engl Ed) 2019;72:972-5. [Crossref] [PubMed]
- Colli A, Besola L, Bizzotto E, et al. Edge-to-edge mitral valve repair with transapical neochord implantation. J Thorac Cardiovasc Surg 2018;156:144-8. [Crossref] [PubMed]
- Salizzoni S, Marro M, Vairo A, et al. Transventricular off-pump anterior mitral leaflet augmentation: First in human. J Thorac Cardiovasc Surg 2019;158:e133-5. [Crossref] [PubMed]







