Surgical versus transcatheter mitral valve replacement in functional mitral valve regurgitation
Introduction
Functional (or secondary) mitral regurgitation (FMR) is a pathologic process that occurs due to anatomic aberrancies of the left ventricle (LV), subvalvular apparatus, or left atrium in the setting of structurally normal valve leaflets (1). Causes of FMR are further subclassified as ischemic (the result of myocardial injury and adverse LV remodeling) or non-ischemic (2). Non-ischemic causes include left atrial dilation secondary to long-standing atrial fibrillation or left ventricular enlargement due to dilated cardiomyopathy. The majority of FMR is ischemic in nature (66%), with the remaining being attributed to dilated cardiomyopathy (3). Overall mortality worsens the longer mitral regurgitation (MR) exists, true for both ischemic and non-ischemic MR (4,5). With worsening MR, maladaptation of ventricular geometry can further beget MR through annular dilation as well as ongoing LV dysfunction, causing malcoaptation of valve leaflets (5-7). Myocardial fibrosis leads to worsening LV function further perpetuating the pathophysiologic state (1,3). Overall prognosis is worse when interventions are performed after symptoms have developed (1,5,6). Poor outcomes for ischemic mitral regurgitation (IMR) are present at even more restrictive criteria as compared to primary MR, effective regurgitant orifice (ERO) ≥0.2 vs. 0.4 cm2, respectively (4). Prior to the results from the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, surgical intervention has been the accepted approach for symptomatic FMR refractory over medical therapy despite only the use of retrospective data. With the advent of transcatheter devices, patients at high surgical risk have demonstrated benefit in early clinical trials.
Herein, we discuss the current evidence and recommendations for surgical and transcatheter replacement approaches to treat FMR. Of note, transcatheter repair options will be discussed elsewhere in this issue of the Journal.
Current guidelines for management of FMR
Indications for surgery
The decision tree for surgical or transcatheter intervention for primary MR is robust and includes class I and IIa level evidence for guiding treatment strategy (8,9). For FMR, the data is not as extensive. Numerous retrospective analyses of FMR have guided treatment strategy for many years, however recent multicenter randomized control trials (RCTs) are challenging preconceived notions about the disease. It is well established that all patients should be on maximum goal-directed medical therapy (GDMT) including: beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, aldosterone antagonists, angiotensin receptor blockers with neprilysin inhibitors, hydralazine/isosorbide dinitrate, coronary revascularization and cardiac resynchronization therapy (8,10,11). Mitral valve surgery has received only a class IIb indication for severe, secondary MR patients with persistent New York Heart Association (NYHA) class III–IV heart failure symptoms (8,10,11). The 2014 American Heart Association (AHA)/American College of Cardiology (ACC) recommendations state mitral valve surgery is reasonable for patients with severe secondary MR (stage C and D) who are undergoing concomitant coronary artery bypass grafting (CABG) or aortic valve replacement (AVR). This was expanded upon in the 2017 AHA/ACC Valvular disease guideline update to include choosing chordal-sparing mitral valve replacement (MVR) over downsized annuloplasty ring based on recent RCT findings (8,12,13).
Surgical mitral valve replacement (SMVR) for FMR
The body of literature concerning severe IMR was primarily comprised of retrospective, single institution analyses, many of which demonstrated that mitral valve repair (MVr) is better for overall outcomes than MVR. The Cardiothoracic Surgical Trials Network (CTSN) investigators sought to clarify and further examine this controversial topic. A total of 251 patients with severe IMR were randomly assigned to undergo MVr or chordal-sparing mitral replacement with or without CABG (11,12). The primary outcome was left ventricular end systolic volume index (LVESVI) at 12 months which for surviving MVr patients was 54.6±25.0 and 60.7±31.5 mL/m2 for the MVR patients equating to a −6.6 and −6.8 mL/m2 difference from baseline, respectively. However, there was no statistical significance in the differences in LVESVI between repair or replacement groups. The death rate was 14.3% for the repair group and 17.6% for the replacement group, but this too was an insignificant difference. Recurrence of moderate or severe MR at 12 months was significantly greater for those in the repair group vs. replacement [32.6% (28.4% moderate, 4.2% severe) vs. 2.3% (all moderate); P<0.001] (11,12). Furthermore, there was a significant difference in LVESVI within the repair group who had recurrent moderate or greater MR at 12 months vs. those without (64.1±23.9 vs. 47.3±23.0 mL/m2; P<0.001). There were no significant differences in death, major adverse cardiac and cerebrovascular events (MACCE), or other serious adverse events (heart failure, arrhythmias, respiratory failure, infections) between the groups at one year. Similarly, there were no significant differences between groups in quality of life (QOL) measures, but there was a superior reduction in heart failure symptoms in patients who received replacement (61.2% vs. 46.9%) (12).
At two years, LVESVI for surviving mitral repair patients was 52.6±27.7 and 60.6±39.0 mL/m2 for the mitral replacement patients equating to a −9.0 and −6.5 mL/m2 from baseline, respectively (13). For both groups, most of the improvement was seen during the first postoperative year. No significant difference of left ventricular ejection fraction (LVEF) existed between the groups at the study’s conclusion. Similarly, cumulative mortality was insignificantly different between the two arms (19.0% repair vs. 23.2% replacement, hazard ratio 0.79, 95% CI: 0.46 to 1.35; P=0.39). The recurrence of moderate or severe MR at 24 months was even more profound than at one year. The repair group exhibited 58.8% recurrence vs. 3.8% in the replacement group (P<0.001). However, just as with the one-year results, the repair patients without moderate or severe MR recurrence had a significantly greater degree of reverse remodeling (LVESVI 42.7±26.4 vs. 62.6±26.9; P<0.001). To summarize, there was a significant difference in relative risk comparing those who underwent mitral repair regarding death, MR recurrence, or need for reintervention (relative risk 2.3; 95% CI: 1.69 to 3.22; P<0.001). Heart-failure related events and cardiovascular readmissions were both significantly higher in the repair group at two years. QOL scores were improved for those undergoing replacement although this did not reach statistical significance. Importantly, the patients who did not experience recurrent MR had significantly improved Minnesota Living with Heart Failure QOL scores (26.6 vs. 16.2 points improvement from baseline; P=0.04) (13).
Given the substantial recurrence of moderate or greater MR, further investigation to identify predictive factors of recurrence were examined (14). Of the 126 patients randomized to the repair arm of this trial, 116 were eligible for inclusion in this analysis. Six patients ultimately were converted to replacement because the initial repair did not adequately correct the MR. The recurrence rates of MR demonstrated on transthoracic echocardiography (TTE) were: 24.8% and 3.0% at 30 days, 25.5% and 4.3% at six months, 29.7% and 4.4% at 12 months and 39.0% and 1.3% at 24 months for moderate and severe MR, respectively. The primary mechanism of MR recurrence was leaflet tethering although no baseline measurements of valvular tethering were associated with moderate or severe MR recurrence. There was, however, a strong association between basal aneurysms and MR recurrence. The authors developed a prediction model for creating a risk score calculator for patients who would go on to develop recurrent MR and may benefit from replacement or advanced repair techniques at their index operation.
In a 2012 study from San Raffaele University Hospital, Milan, Italy, a retrospective analysis was conducted in 132 patients undergoing MVr vs. replacement for dilated and ischemic cardiomyopathy (15). The etiology of the MR was ischemic in 89 (67.4%) of cases. MVr was performed in 85 patients (64.4%). All patients had moderately-severe (3+/4+) or severe (4+/4+) MR. Demographic, preoperative comorbidity and echocardiographic parameters were similar between the two groups with the exception of preoperative LVEF in the repair group which was significantly lower (30.8%±7.71% vs. 33.6%±7.69%; P=0.04). The surgical procedure was chosen based on preoperative echocardiographic data. MVRs were performed when one or more of the following criteria were identified: absence or mild dilation of the mitral annulus, complex multiple regurgitant jets, extreme tethering of posterior leaflet (leaflet-annular plane angle >45 degrees) and excess bileaflet tethering (tenting area >4 cm2, coaptation depth >18 mm). Mitral repairs all utilized a rigid or semirigid annuloplasty ring. All replacements were performed with chordal-sparing technique and preservation of the subvalvular apparatus. Concomitant procedures including CABG, tricuspid annuloplasty, and radiofrequency ablation for atrial fibrillation were similar between the two groups. Thirty-day mortality was significantly different between the two groups (MVr 2.3% vs. MVR 12.7%; P=0.03) with replacement being the only predictor of mortality on univariate analysis (odds ratio 6, 95% CI: 1.2 to 31, P=0.03). Overall survival at 2.5 years favored patients undergoing repair vs. replacement (92%±3.2% vs. 73%±7.9%, P=0.02). Similarly, the only predictor of overall mortality on univariate analysis was MVR (hazard ratio 3.1, 95% CI: 1.1 to 8.9, P=0.02). Clinically, the repair group (30.8%±7.7% to 38.2%±9.5%; P<0.0001) outperformed the replacement group (33.6±7.6 to 31.4±9.8; P=0.5) in LVEF improvement, as well as markers of reverse LV remodeling, LVEDV and LVESV (P=0.0001). Overall reverse remodeling at follow-up was demonstrated by 37/83 (44.6%) repair patients and 4/41 (9.8%) of patients undergoing MV replacement (P=0.0002). MR graded moderate or worse was present in 18/83 (21.7%) of the repair patients, all of whom did not meet echocardiographic parameters to be considered to have reverse LV remodeling. Functionally, NYHA Class improved dramatically, again favoring the repair group (P=0.06), but for the cohort, Class 3 or 4 symptoms were present in only 9/124 (7.3%; P=0.0001) of patients who survived to follow-up.
The American Association for Thoracic Surgery has generated its own consensus guidelines based on the latest randomized clinical trials, observational studies and the expert opinions of the panel (11). The recent 2016 update in response to the CTSN trials for severe and moderate IMR expand upon or clarify the AHA/ACC guidelines. Specifically, for patients with moderate IMR, they suggest in patients undergoing CABG, MVr with an undersized, complete annuloplasty ring may be considered. Furthermore, for severe IMR, replacement is reasonable for symptomatic patients, and those with a basal aneurysm or dyskinesis, significant leaflet tethering, and/or severe left ventricular dilation [end diastolic diameter (EDD) >6.5 cm]. For patients with severe IMR, MVr may be considered with an undersized complete annuloplasty ring that does not meet the above anatomic criteria. In the context of MVr vs. replacement, MVR should be performed with preservation of anterior and posterior leaflet cords (16), while MVr should utilize an undersized complete rigid/semirigid annuloplasty ring (17).
Transcatheter mitral valve replacement (TMVR)
Since the advent of transcatheter mitral valve repair, industry colleagues have developed many devices in the pursuit of TMVR (see Figure 1). Although MVr is often preferred, particular anatomy and mitral disease may require replacement (5). For some time, many centers were using the Edwards Sapien transcatheter heart valves (Edwards Lifesciences Corp., Irvine, California) for TMVR (18). This off-label approach is utilized in degenerated mitral valve surgical prostheses or within annuloplasty rings via a transapical or transseptal approach (18,19). The increasing use of transcatheter devices in patients deemed too high-risk for surgery led to the question of efficacy in patients with FMR. With the feasibility of TMVR proven in off-label cases, there is great enthusiasm and investment in transcatheter mitral replacement devices for use in native mitral valves. However, the complexity of the mitral valve, varied etiology of pathology, and complex mechanisms of mitral valve disease have stymied the rapid development of TMVR (20). Some other factors that have slowed development are as follows: anatomic variability of the mitral annulus, mitral annular calcification (MAC), complexity and variability of the subvalvular apparatus, asymmetry of the mitral annulus, and absence of a single valvular plane (21). Unlike the aortic annulus, the mitral annulus and basal portion of the LV are in constant motion, making stable seating of the prosthesis challenging (21). The proximity of the mitral valve to the aortic valve and left ventricular outflow tract (LVOT) can create obstruction and is one of the most common concerns with TMVR (22,23). With these challenges in mind, there have been numerous transcatheter valves that have been designed for de novo mitral valve disease with variable results as the therapy has been utilized in early human trials.

A global feasibility trial: Tendyne
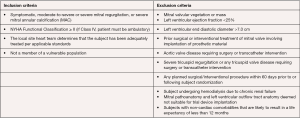
The Global Feasibility Study for Tendyne (Tendyne Holdings, LLC, Roseville, Minnesota—a subsidiary of Abbott Vascular) enrolled patients at eight study sites in the United States, Australia, and Norway between November 2014 and March 2016 (24,25). Inclusion criteria for the study were: age ≥18 years, MR grade 3 or 4 (primary or secondary), symptoms of dyspnea (NYHA Class ≥ II), and ability to provide informed consent (see Figure 2). Exclusion criteria were: LV end-diastolic diameter >70 mm, severe mitral annular or leaflet calcification, left atrial or LV thrombus, prior mitral or aortic valve surgery, prior transcatheter mitral intervention, pulmonary artery systolic pressure >70 mmHg, severe tricuspid regurgitation, and severe right ventricular dysfunction with evidence of right heart failure (24-26).
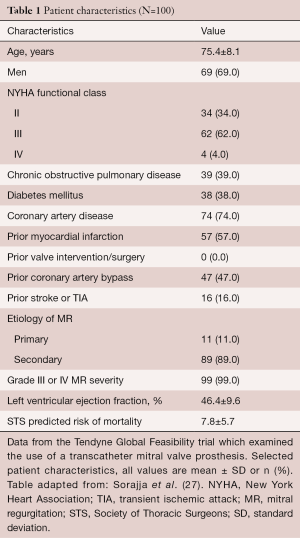
The first 30 patients (age 75.6±9.2 years) treated with Tendyne included 25 men (Table 1). The majority of the patients [23/30] had secondary MR due to ischemic chordal tethering or failure of leaflet coaptation (24,25). During initial hospital stay, only one patient died and this was due to hospital acquired pneumonia and respiratory failure. There were no strokes, myocardial infarctions, or additional device related complications during hospitalization. At discharge, 65.5% of patients went home and the remainder transferred to a skilled nursing facility. At 30 days, no additional deaths were reported and follow-up TTE demonstrated MR grade 0 in all but one patient who had mild (1+) centrally directed regurgitation. No patient was subject to device migration, embolization or mitral surgery. This study demonstrated that TMVR can be effective and safe for selected high-risk patients with symptomatic secondary severe MR. The major limitations of this study were that it was a non-randomized study without a control group with a relatively small patient cohort.
Full table
More recently, the results of the first 100 patients in Global Feasibility Tendyne Trial have been reported which included the first 30 patients reported above. The mean age was 75.4±8.1 years and included 69 men and 31 women. Once again, the majority of the patients [89/100] had secondary MR. Advanced heart failure symptoms (66%) defined as NYHA Class III or IV was common. Most patients had left ventricular dysfunction (mean ejection fraction, 46.4%±9.6%), and significant comorbidities (Society of Thoracic Surgeons Predicted Risk of Mortality, 7.8%±5.7%) (25). There were no intraprocedural deaths, one instance of major apical bleeding, and no acute conversion to conventional surgery or need for cardiopulmonary bypass. Technical success was 96%. Reasons for technical failure included LVOT obstruction, malpositioned device, poor apical access and hemodynamic instability during guidewire displacement. The 30-day rates of mortality and stroke were 6% and 2%, respectively. The one-year survival free of all-cause mortality was 72.4% (95% CI: 62.1% to 80.4%), with 84.6% of deaths due to cardiac causes (25). Among survivors at one year, 88.5% were NYHA function Class I/II, and demonstrated improvements in six-minute walk distance (P<0.0001) and quality-of-life measurements (P=0.011). Investigators in the Tendyne Global Feasibility Trial concluded that TMVR can be performed safely, is a durable correction of MR at one-year follow-up, and significantly improved patients’ symptoms.
A global feasibility trial: Intrepid
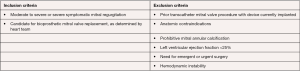
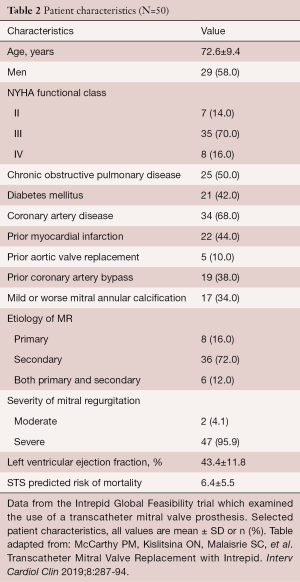
The Intrepid (Medtronic, Minneapolis, MN, USA) Global Feasibility Trial enrolled 50 patients from 14 study sites in the United States, Australia, and Europe. Inclusion criteria for the study were: symptomatic severe MR, high or extreme surgical risk as determined by the local heart team, mitral valve geometry and size compatible with the available intrepid sizes, mild or no mitral valve calcification and an LV ejection fraction greater than or equal to 20% (see Figure 3) (27). Patients were excluded with evidence of severe pulmonary hypertension (systolic PAP >70 mmHg), need for coronary revascularization, hemodynamic instability, need for other valve therapy, renal insufficiency (serum creatinine >2.5 mg/dL), and prior mitral valve surgery or intervention. Severe heart failure (NYHA Class III or IV) was common among the study population (86%) (27). Major comorbidities such as diabetes, coronary artery disease and atrial fibrillation were also common (see Table 2). All patients were high surgical risk with overall Society of Thoracic Surgeons Predicted Risk of Mortality 6.4%±5.5%. The predominant etiology MR was secondary in 84%. A total of 48 out of 50 patients underwent successful implantation of the valve (27). Of the two patients that did not have successful valve implantation, one was due to LV apical bleeding, and the other due to sizing miscalculation and malpositioning of the valve. There was a 30-day mortality rate of 14% (n=7) (27). Causes of death included bleeding complications at apical access site (n=3), refractory heart failure (n=3) and malposition of valve implant (n=1). Patients alive at 30 days (n=42) demonstrated significant reduction in the severity of MR and symptom improvement. Echocardiography at follow-up demonstrated either absent or mild MR for all survivors. This initial trial showed the Intrepid TMVR device can be successfully implanted making it a promising approach for patients who are high surgical risk.
Full table
Ongoing clinical trials for TMVR
SUMMIT trial
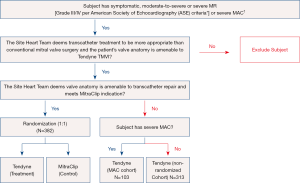
The SUMMIT pivotal trial is designed to evaluate the safety and effectiveness of using the Tendyne mitral valve system for the treatment of symptomatic mitral regurgitation (28). There are three trial cohorts: randomized, non-randomized and the MAC arm (see Figure 4). Patients in whom the heart team determines a transcatheter approach is preferred over a surgical approach are eligible. In patients with suitable anatomy for transcatheter repair with MitraClip (Abbott Vascular, Santa Clara, CA), patients are randomized in a 1:1 ratio to the Tendyne device or to MitraClip after GDMT is achieved. In patients who are not anatomically amenable for MitraClip, they can be enrolled in a non-randomized arm. Finally, patients with severe MAC who are not suitable for MitraClip and at high-risk for surgery, they can be treated in the MAC arm. The primary outcome measure for the randomized cohort is survival free of heart failure hospitalization at 12 months post index procedure (28). The non-randomized cohort and the MAC cohort primary outcomes of interest are all-cause mortality, cardiovascular related hospitalizations, stroke or mitral valve reintervention/reoperation and survival free of heart failure hospitalization at twelve months post index procedure.
APOLLO trial
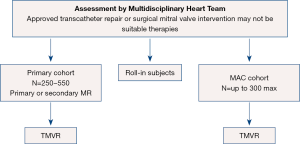
The APOLLO pivotal trial is designed to evaluate the safety and effectiveness of using the Medtronic Intrepid TMVR System in patients with severe MR who require MVR. There are two arms to the APOLLO trial (see Figure 5) including a primary cohort (primary or secondary MR) and a MAC arm with a total of 250–550 subjects in the primary cohort and up to 300 subjects in the MAC cohort (27). In contrast to SUMMIT, all subjects will undergo TMVR. Primary endpoints for the primary cohort include a composite of all-cause mortality or heart failure hospitalization post-30 days or Kansas City Cardiomyopathy Questionnaire (KCCQ) improvement <10 points at one year (27). The single-arm MAC cohort primary outcomes at one year are all-cause mortality and heart failure hospitalization.
Edwards Evoque TMVR early feasibility study
The Evoque early feasibility trial is a single-arm, non-randomized study which seeks to evaluate the safety and function of the Evoque TMVR device (29). There are 17 enrollment sites across the United States and Canada. Primary outcome of interest is safety which is assessed by freedom from device or procedure-related complications within 30 days (29). Secondary outcomes are measures of symptomatic improvement (NYHA Class and six-minute walk test) along with reduction in MR grade. Secondary outcomes will be measured at 30 days, three, six, twelve months and annually for five years. All study participants must be 18 years of age or older (Figure 6) with clinically significant MR and at high risk for surgery with suitable anatomy (29). Enrollment thus far has been limited. Early results of 14 patients demonstrate procedural success in 93% (n=13) with one conversion to surgery. At 30-day follow-up, one patient died. In survivors, MR was eliminated in 80% of patients and reduced to 1+ in 20% of patients (29).
Conclusions
MVR has a role, particularly in the treatment of patients with functional MR. The advent of transcatheter devices has expanded options for MVR in patients who are high-risk candidates for traditional surgery. TMVR has promising results when assessing valve function and QOL improvement at 30-day and 1- and 2-year follow-up. Furthermore, early results of the SUMMIT, Apollo, and Evoque trials have demonstrated safety, reduction of MR and symptom improvement. These studies will further guide clinical practice about how to best employ TMVR and SMVR in patients with FMR. Finally, transcatheter repair and replacement technologies will need to be investigated to determine which therapy is best for a given patient. In summary, this is an extremely exciting time in the rapidly evolving field of mitral therapies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: DSL, MD: Research grants: Edwards, Abbott; Gorav Ailawadi, MD, MBA: Consulting: Medtronic, Admedus, Gore, Edwards, Abbott, Atricure (all <5K). The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chehab O, Roberts-Thomson R, Ng Yin Ling C, et al. Secondary mitral regurgitation: pathophysiology, proportionality and prognosis. Heart 2020;106:716-23. [Crossref] [PubMed]
- Krawczyk-Ożóg A, Holda MK, Bolechala F, et al. Anatomy of the mitral subvalvular apparatus. J Thorac Cardiovasc Surg 2018;155:2002-10. [Crossref] [PubMed]
- Yousefnia MA, Dehestani A, Saidi B, et al. Papillary muscle repositioning in valve replacement for left ventricular dysfunction: ischemic mitral regurgitation. Ann Thorac Surg 2010;90:497-502. [Crossref] [PubMed]
- Yamaguchi A, Adachi K, Yuri K, et al. Reduction of mitral valve leaflet tethering by procedures targeting the subvalvular apparatus in addition to mitral annuloplasty. Circ J 2013;77:1461-5. [Crossref] [PubMed]
- Del Val D, Ferreira-Neto AN, Wintzer-Wehekind J, et al. Early Experience With Transcatheter Mitral Valve Replacement: A Systematic Review. J Am Heart Assoc 2019;8:e013332. [Crossref] [PubMed]
- Borger MA. Ischemic Mitral Regurgitation: Why, When, and How? CTSNet, Inc., 2017. Retrieved: 19:27, Nov 30, 2017 (GMT). Available online: https://doi.org/ [Crossref]
- Gillinov AM. Is ischemic mitral regurgitation an indication for surgical repair or replacement? Heart Fail Rev 2006;11:231-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]
- Giustino G, Overbey J, Taylor D, et al. Sex-Based Differences in Outcomes After Mitral Valve Surgery for Severe Ischemic Mitral Regurgitation: From the Cardiothoracic Surgical Trials Network. JACC Heart Fail 2019;7:481-90. [Crossref] [PubMed]
- American Association for Thoracic Surgery Ischemic Mitral Regurgitation Consensus Guidelines Writing Committee. 2016 update to The American Association for Thoracic Surgery consensus guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg 2017;153:1076-9. [Crossref] [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1. [Crossref] [PubMed]
- De Bonis M, Ferrara D, Taramasso M, et al. Mitral replacement or repair for functional mitral regurgitation in dilated and ischemic cardiomyopathy: is it really the same? Ann Thorac Surg 2012;94:44-51. [Crossref] [PubMed]
- Yun KL, Sintek CF, Miller DC, et al. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg 2002;123:707-14. [Crossref] [PubMed]
- Silberman S, Klutstein MW, Sabag T, et al. Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg 2009;87:1721-6; discussion 1726-7. [Crossref] [PubMed]
- Testa L, Popolo Rubbio A, Casenghi M, et al. Transcatheter Mitral Valve Replacement in the Transcatheter Aortic Valve Replacement Era. J Am Heart Assoc 2019;8:e013352. [Crossref] [PubMed]
- Beller JP, Rogers JH, Thourani VH, et al. Early clinical results with the Tendyne transcatheter mitral valve replacement system. Ann Cardiothorac Surg 2018;7:776-9. [Crossref] [PubMed]
- Regueiro A, Granada JF, Dagenais F, et al. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J Am Coll Cardiol 2017;69:2175-92. [Crossref] [PubMed]
- Ramlawi B, Gammie JS. Mitral Valve Surgery: Current Minimally Invasive and Transcatheter Options. Methodist Debakey Cardiovasc J 2016;12:20-6. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2019;12:182-93. [Crossref] [PubMed]
- Wang DD, Eng M, Greenbaum A, et al. Predicting LVOT Obstruction After TMVR. JACC Cardiovasc Imaging 2016;9:1349-52. [Crossref] [PubMed]
- Sorajja P, Gossl M, Babaliaros V, et al. Novel Transcatheter Mitral Valve Prosthesis for Patients with Severe Mitral Annular Calcification. J Am Coll Cardiol 2019;74:1431-40. [Crossref] [PubMed]
- Sorajja P, Moat N, Badhwar V, et al. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J Am Coll Cardiol 2019;73:1250-60. [Crossref] [PubMed]
- Niikura H, Gossl M, Sorajja P. Transcatheter Mitral Valve Replacement with Tendyne. Interv Cardiol Clin 2019;8:295-300. [Crossref] [PubMed]
- U.S National Library of Medicine. Transcatheter Mitral Valve Replacement with the Medtronic IntrepidTM TMVR System in Patients with Severe Symptomatic Mitral Regurgitation (APOLLO). 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03242642
- U.S National Library of Medicine. Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation (SUMMIT). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03433274
- Webb J, Hensey M, Fam N, et al. Transcatheter Mitral Valve Replacement with the Transseptal EVOQUE System. JACC Cardiovasc Interv 2020;13:2418-26. [Crossref] [PubMed]