LAMPOON techniques to prevent or manage left ventricular outflow tract obstruction in transcatheter mitral valve replacement
Introduction
Mitral valve disease is a leading global cause of morbidity and mortality (1,2). Many patients are unsuitable for surgical mitral valve repair or replacement due to a high predicted risk of mortality (3,4). In a subset of high-risk patients with suitable anatomy, transcatheter mitral valve replacement (TMVR) may be a therapeutic option (5). Pending the approval of dedicated devices, transcatheter aortic valves may be implanted in the mitral position in a bioprosthetic valve (valve-in-valve), mitral annuloplasty ring (valve-in-ring) or in native mitral annular calcification (valve-in-MAC) (6,7). In-hospital, thirty-day and one-year mortality rates are highest for valve-in-MAC as compared to valve-in-ring or in valve-in-valve (6,7).
The close relationships between the left ventricular outflow tract (LVOT) and the anterior mitral valve leaflet can lead to LVOT obstruction. The incidence of LVOT obstruction in TMVR occurs in up to 10–40% of valve-in-MAC, 5% of valve-in-ring, and 0.7–2% of valve-in-valve cases (6,7). When this phenomenon occurs, outcomes are poor, with in-hospital mortality rates as high as 62% (8). Furthermore, there is a high percentage screening failure, estimated to be as high as 89%, with the most common exclusion criteria being excessive frailty, severe tricuspid regurgitation, right ventricular dysfunction, prior aortic valve therapy, severe MAC and risk for LVOT obstruction (9,10). The use of a multidisciplinary heart team to assess all these patient and procedural factors are important when deciding when to perform a TMVR procedure. It is important to predict the risk of LVOT obstruction after TMVR and to use appropriate techniques to prevent this complication. In this review, we discuss predictors of LVOT obstruction, the use of Laceration of the Anterior Mitral leaflet to Prevent Outflow ObtructioN (LAMPOON) to prevent LVOT obstruction, and LAMPOON as a bail-out technique to treat TMVR-induced LVOT obstruction.
LVOT obstruction
The Mitral Valve Academic Research Consortium defines LVOT obstruction as a sudden decline in hemodynamics following TMVR with intra-procedural transesophageal echocardiographic (TEE) images showing displacement of prosthetic or anterior native mitral valve leaflet obstructing LVOT and an increase in LVOT gradient by 10 mmHg (11,12). However, clinically significant LVOT obstruction is more likely when gradients exceed 30 mmHg (13). Two main mechanisms have been described. “Fixed obstruction” (sometimes referred to as “geometric obstruction”) is due to a narrowed and elongated neo-LVOT, caused by the anterior mitral leaflet being pushed towards the interventricular septum from the new transcatheter valve. “Dynamic obstruction” is due to systolic anterior motion (SAM) of the anterior mitral leaflet towards the interventricular septum during systole, due to Bernoulli forces generated by the neo-LVOT (13).
Predicting LVOT obstruction
“Fixed obstruction” can be predicted by calculating a “neo-LVOT” on computed tomography (CT) reconstruction. The neo-LVOT is the smallest cross-sectional area circumscribed by the transcatheter heart valve (THV) and the left ventricular septum, and it also contributes to post-procedural LVOT gradient. The neo-LVOT can be predicted on multi-slice cardiac CT imaging by simulating a virtual THV and measuring the projected minimal cross-sectional area (14). Observational studies suggest that a neo-LVOT area of less than 170–190 mm2 confers increased risk of LVOT obstruction (11). In addition, the “skirt” neo-LVOT can be measured using the area by the THV-covered cells and the interventricular septum, thus simulating the neo-LVOT area if the anterior mitral leaflet was removed surgically or parted by LAMPOON (15).
“Dynamic obstruction” from SAM of the anterior mitral leaflet is harder to predict. Acute aortomitral angulation, reduced mitral annulus-to-interventricular septum (IVS) distance, prominent septal bulge, long anterior mitral leaflet, and redundant mitral chordae are all likely factors that will increase risk. Also, a long anterior mitral leaflet may prolapse back into the THV, interfering with valve closure and ultimately resulting in acute valve failure (14).
LAMPOON
LAMPOON mimics the surgical technique of resection of the anterior mitral leaflet to prevent LVOT obstruction (16,17). The split anterior mitral leaflet parts away from the LVOT and blood flow, is maintained through the open cells of the THV, despite threatened obstruction were the anterior mitral leaflet intact. In addition, the technique spares the subvalvular apparatus in order to preserve left ventricular function. In the procedure, the anterior mitral valve leaflet is split down the midline using focused radiofrequency energy directed by catheters and guidewires. There are several iterations of the LAMPOON technique, each appropriate in different settings (Central Illustration). The principles of transcatheter electrosurgery, enabling leaflet traversal and precise laceration, have been extensively explored in a recent state-of-the-art review (18). The sections below illustrate these techniques and their appropriate scenarios.
Retrograde “Classic” LAMPOON (Figures 1,2)
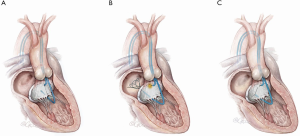
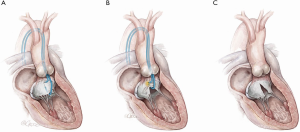
This is this original iteration of the LAMPOON technique (16,17). The procedure steps are illustrated and described in Figures 1,2. The LAMPOON IDE study demonstrated that the LAMPOON procedure was feasible in 100% of subjects (n=30) across a variety of native and annuloplasty risk anatomies and calcium patterns (13). The in-hospital survival rate was 93% and thirty-day survival rate was 100% valve-in-ring and 87% valve-in-MAC.
There were no strokes in the study. The trial confirmed that LAMPOON is feasible in native and annuloplasty ring anatomies in patients who were otherwise ineligible for treatment, and with acceptable safety (13). The advantage of retrograde LAMPOON is that the vector of laceration aligns along the LVOT. The main disadvantage is the difficulty in positioning the traversal guide underneath the A2-scallop of the anterior mitral valve leaflet. Retrograde LAMPOON is not suitable in patients with mechanical aortic valve bioprostheses, due to the likely hemodynamic instability caused by two guiding catheters across the mechanical aortic valve.
Antegrade LAMPOON (Figure 3)
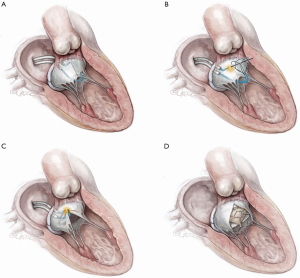
The most technically challenging step in retrograde LAMPOON is positioning the retrograde guide catheter at the base of A2. To simplify the procedure, an antegrade approach was developed. The antegrade approach also enables LAMPOON to work in the setting of a mechanical aortic valve. The procedure steps are described in Figure 3. The initial preliminary results (n=8) demonstrated that the technique was successful in 100% of patients with a minimal increase in LVOT gradient post-TMVR ≤10 mmHg. In addition, no patients experienced an insufficient laceration or significant hemodynamic compromise and all patients survived the procedure. The median wire traversal-to-leaflet laceration time was twenty-six minutes, median laceration-to-valve implantation time was eighteen minutes and median number of attempts at successful leaflet traversal was one minute. This case series suggests that antegrade LAMPOON is an effective, reproducible and simplified strategy to lacerate the anterior mitral leaflet (AML) prior to TMVR (17). Compared with retrograde LAMPOON, this approach offers greater guide catheter stability and more precise positioning over the target A2 scallop. The main disadvantage is the risk of eccentric mitral valve laceration from the center of A2 medially towards the interatrial septum. A stable pivot provided by the two steerable sheaths is essential to avoid eccentric laceration.
Tip-to-base LAMPOON (Figure 4)
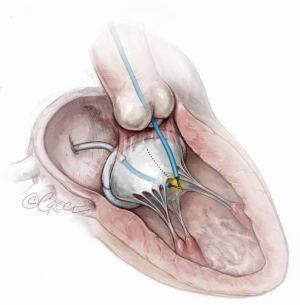
When the aorto-mitral curtain is protected by a complete bioprosthetic ring or bioprosthetic mitral valve sewing ring, LAMPOON laceration may be performed from leaflet tip to base, obviating the need for leaflet traversal. This iteration is another major step in simplifying the technique in this subset of patients. These procedure steps are illustrated in Figure 4. For select patients with high-risk anatomy, undergoing ViV or valve-in-(complete)-ring TMVR, tip-to-base LAMPOON appears to be an effective solution to prevent LVOT obstruction (19). The major advantage of tip-to-base LAMPOON is the simplicity afforded by eliminating the traversal step. It is only appropriate in cases where there is an adequate “backstop” to prevent laceration extending into the aorto-mitral curtain and aortic valve. The disadvantages involved include insufficient laceration of the leaflet and inadvertent laceration of adjacent structures, if care is not taken to insulate the active cutting surface adequately.
“Rescue” LAMPOON (Figure 5)
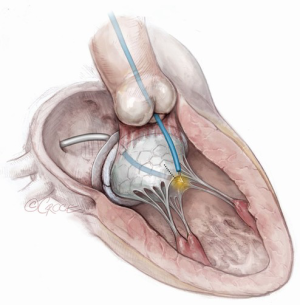
In cases where there is SAM causing LVOT obstruction after TMVR, LAMPOON can be performed to lacerate the protruding native anterior mitral valve leaflet (20). The technique is similar to when performing “tip-to-base” LAMPOON and is illustrated in Figure 5. This is a useful technique because cases of LVOT obstruction from SAM in the setting of a generous neo-LVOT is difficult to predict, and this treatment can be used as a bail-out and an alternative to alcohol septal ablation. This should not be used as a substitute for improving predictors of dynamic LVOT obstruction.
Conclusions
LVOT obstruction after TMVR continues to be a major concern and carries a high risk of in-patient mortality. LAMPOON is a versatile technique that has been prospectively studied in an early feasibility clinical trial. It enables TMVR in patients who would otherwise be ineligible due to high risk of LVOT obstruction. Iterations of the LAMPOON technique have been developed and may make the procedure easier to perform in certain anatomies.
Acknowledgments
Funding: Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, USA (Z01-HL006040).
Footnote
Conflicts of Interest: VCB is a consultant for Edwards Lifesciences and for Abbott Vascular, and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific. ABG is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular. He is a consultant for Transmural Systems. TR is a consultant/proctor for Medtronic and Edwards Lifesciences. He has equity shares in Transmural Systems. RW—Advisory Board: Abbott Vascular, Amgen, Boston Scientific, Medtronic, Philips, Pi-Cardia Ltd., Cardioset; Consultant: Abbott Vascular, Amgen, Biosensors, Biotronik, Boston Scientific, Medtronic, Philips, Pi-Cardia Ltd., Cardioset; Grant Support: Abbott Vascular, AstraZeneca, Biosensors, Biotronik, Boston Scientific, Chiesi; Speakers Bureau: AstraZeneca, Chiesi; Investor: MedAlliance. JMK is a proctor for Edwards Lifesciences and Medtronic. JMK, TR, and RJL are co-inventors on patents, assigned to NIH, on catheter devices to lacerate valve leaflets. NHLBI has a collaborative research and development agreement with Edwards Lifesciences on transcatheter modification of the mitral valve. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart 2016;102:75-85. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [Crossref] [PubMed]
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. [Crossref] [PubMed]
- Regueiro A, Granada JF, Dagenais F, et al. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J Am Coll Cardiol 2017;69:2175-92. [Crossref] [PubMed]
- Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441-51. [Crossref] [PubMed]
- Guerrero M, Vemulapalli S, Xiang Q, et al. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv 2020;13:e008425. [Crossref] [PubMed]
- Guerrero M, Urena M, Himbert D, et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol 2018;71:1841-53. [Crossref] [PubMed]
- Niikura H, Gossl M, Kshettry V, et al. Causes and Clinical Outcomes of Patients Who Are Ineligible for Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2019;12:196-204. [Crossref] [PubMed]
- Del Val D, Ferreira-Neto AN, Wintzer-Wehekind J, et al. Early Experience With Transcatheter Mitral Valve Replacement: A Systematic Review. J Am Heart Assoc 2019;8:e013332. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2019;12:182-93. [Crossref] [PubMed]
- Stone GW, Adams DH, Abraham WT, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015;66:308-21. [Crossref] [PubMed]
- Khan JM, Babaliaros VC, Greenbaum AB, et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2019;73:2521-34. [Crossref] [PubMed]
- Blanke P, Naoum C, Dvir D, et al. Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation: Concept of the Neo-LVOT. JACC Cardiovasc Imaging 2017;10:482-5. [Crossref] [PubMed]
- Khan JM, Rogers T, Babaliaros VC, et al. Predicting Left Ventricular Outflow Tract Obstruction Despite Anterior Mitral Leaflet Resection: The "Skirt NeoLVOT". JACC Cardiovasc Imaging 2018;11:1356-9. [Crossref] [PubMed]
- Khan JM, Rogers T, Schenke WH, et al. Intentional Laceration of the Anterior Mitral Valve Leaflet to Prevent Left Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement: Pre-Clinical Findings. JACC Cardiovasc Interv 2016;9:1835-43. [Crossref] [PubMed]
- Babaliaros VC, Greenbaum AB, Khan JM, et al. Intentional Percutaneous Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction During Transcatheter Mitral Valve Replacement: First-in-Human Experience. JACC Cardiovasc Interv 2017;10:798-809. [Crossref] [PubMed]
- Khan JM, Rogers T, Greenbaum AB, et al. Transcatheter Electrosurgery: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:1455-70. [Crossref] [PubMed]
- Case BC, Khan JM, Satler LF, et al. Tip-to-Base LAMPOON to Prevent Left Ventricular Outflow Tract Obstruction in Valve-in-Valve Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv 2020;13:1126-8. [Crossref] [PubMed]
- Khan JM, Trivedi U, Gomes A, et al. "Rescue" LAMPOON to Treat Transcatheter Mitral Valve Replacement-Associated Left Ventricular Outflow Tract Obstruction. JACC Cardiovasc Interv 2019;12:1283-4. [Crossref] [PubMed]






