Concomitant cardiac surgery procedures during left ventricular assist device implantation: single-centre experience
Introduction
Left ventricular assist device (LVAD) implantation as bridge to transplantation, recovery or destination therapy (DT) is a standard treatment for patients with end-stage heart failure (1). Additional cardiac pathologies, especially valvular ones, are common in advanced heart failure with a reported prevalence of 30% to 50% for tricuspid valves, 40% to 70% for mitral valves and 20% for the aortic valve (2-5). It remains controversial whether issues other than more than mild aortic valve regurgitation or intracardiac shunts [both Class I indications for concomitant surgery according to the International Society of Heart and Lung Transplantation (ISHLT) guidelines (6)] should be addressed at the time of LVAD implantation. Multiple studies have shown contrary results with both adverse effects and improvement of outcomes in terms of right ventricular (RV) function, length of intensive care unit and hospital stay, catecholamine dependency, mortality and more, after LVAD and concomitant cardiac surgery (7-12).
With this study, we sought to examine our institutional outcomes of adult continuous-flow LVAD recipients with and without additional cardiac procedures over the past fourteen years.
Methods
Study population
Overall 357 consecutive adult patients underwent continuous-flow LVAD implantation between March 2006 to July 2020 at our department. Out of these, a total of 91 patients had a concomitant cardiac procedure at the time of LVAD implantation. Patients undergoing non-valvular procedures additional to LVAD implantation (n=5) were excluded from further analysis. Data was obtained from our department’s Mechanically Circulatory Support database. The study was approved by the local Ethics Committee.
Ventricular assist device systems and implantation techniques
LVAD systems used in the study population were the Medtronic HVAD® (HVAD), the Abbott HeartMate IITM LVAD (HMII) and the HeartMate 3TM LVAD (HM3). Implantations were performed either via standard full sternotomy (FS) or in a less invasive (LIS) fashion. Briefly, the LIS techniques are conducted as follows: For isolated LVAD implantation, bilateral mini-thoracotomy in the fourth or fifth left intercostal space and the second right intercostal space is performed, granting access to the left ventricular apex as well as to the ascending aorta. For combined procedures, a left-sided mini-thoracotomy and an upper hemi-sternotomy in the third intercostal space are performed (13-15).
We perform mitral valve repair (MVR) in high grade secondary insufficiency, primary mitral regurgitation is always addressed. A similar policy is pursued in the management of tricuspid valve regurgitation. The preferred technique is a prosthetic ring annuloplasty. In selected cases, implantation of a biological prosthesis might be necessary. Aortic valve procedures and closure of intracardiac shunts are performed as indicated by the ISHLT guidelines (6).
Outcome measures
Study endpoints were short-term survival (thirty-day mortality and in-hospital mortality), one-year outcomes and the need for temporary RV support.
Statistical analysis
Categorical variables were presented as numbers and percentages. Mean values with standard deviations (SDs) or median values with interquartile ranges (IQRs) were determined for continuous variables. Comparison of means was performed using the paired t-test or Wilcoxon test, when appropriate. Comparison of categorial variables was performed with the Pearson’s χ2 test or Fisher’s exact test, when indicated. A P value <0.05 was considered statistically significant.
Results
Overall cohort
Patient characteristics
Median age was 60 (IQR: 52–66) years, the majority of patients were male (86%) and suffered from ischemic cardiomyopathy (ICMP; 58%). Most patients (n=107) were in INTERMACS level 4 at the time of LVAD implantation (32%), 76 patients were in INTERMACS level 3 (23%), 58 were in INTERMACS level 2 (17%) and 97 were in INTERMACS level 1 (29%). Fifty-eight patients (17%) were on preoperative extracorporeal life support (ECLS). A bridge to candidacy (BTC) strategy was pursued in 260 patients (74%).
Operative procedure
Overall 177 patients received a Medtronic HVAD system (50%), 115 patients a HM3 (33%) and 60 patients a HMII (17%). Isolated LVAD implantation with or without temporary RV support was performed in 266 patients (76%). Twenty-four percent had a concomitant cardiac procedure (other than temporary RV support systems). A LIS implantation technique was carried out in128 patients (36%).
Postoperative outcomes
Median duration of LVAD support was 503 (IQR: 214–967) days. Thirty-day and in-hospital mortality were 6% and 16%, respectively, one-year survival was 77%. After a median follow-up time of 30 (IQR: 9–67) months, 42% (n=149) of the patients were deceased, 32% (n=111) were transplanted, 24% were alive on the device (n=86) and 6 patients (2%) were successfully weaned.
Patients with concomitant cardiac procedures
Patient characteristics and procedural data
The median age of this sub-cohort’s patients was 60 (IQR: 52–67) years, the vast majority were male (80%). Patients with concomitant cardiac procedures suffered from dilatative cardiomyopathy (DCMP) more often than patients undergoing isolated LVAD implantation (53% vs. 36%, P=0.005). There was also a significant difference in the distribution of INTERMACS level: patients receiving additional interventions were more frequently in INTERMACS level 4 (38% vs. 30%, P=0.154), whereas the majority of the isolated LVAD group were in level 1 (32% vs. 16%, P=0.003). Significantly less patients were on preoperative ECLS compared to isolated LVAD procedures (5% vs. 20%, P=0.001).
A BTC concept was pursued in 62 patients (72%), similar to the isolated LVAD group (74%). For more details see Table 1.

Full table
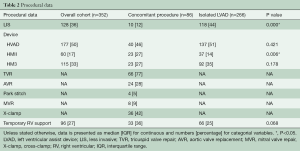
The most common concomitant cardiac procedure was tricuspid valve annuloplasty in 66 patients (77%), followed by aortic valve procedures in 28 patients (33%; bio-prosthesis in 86%, and modified Park’s stitch in 14%). Other procedures included mitral valve annuloplasty (9%) and mitral valve replacement (1%). Overall 18 patients (21%) had two or more cardiac procedures in addition to LVAD implantation.
LIS techniques were carried out in 10 patients (12%) with concomitant cardiac procedures and in 118 patients (44%) without concomitant cardiac procedures (P=0.000).
Mean cardiopulmonary bypass (CPB) times were 163±50 min. Aortic cross-clamping was required in 42% of the cases.
Temporary RV support was necessary in 30 patients (35%), using either ECLS (37%) or a temporary RV assist device (RVAD) to the pulmonary artery (63%). Though not statistically significant, there is still a trend towards a higher need for RV support compared to what was observed in isolated LVADs (25%, P=0.06) (Table 2).
Full table
Upon detailed analysis of the two most frequent concomitant procedures, isolated tricuspid valve repair (TVR) (56%) and isolated aortic valve procedures (18%), we observed similar durations of CPB (163±71 min for aortic procedures vs. 153±39 min for tricuspid repair, P=0.975). Not surprisingly, aortic cross-clamping was necessary in only one case of tricuspid repair and all aortic procedures (2% vs. 100%, P=0.000).
Postoperative outcomes
Median duration of LVAD support was 403 (IQR: 125–1,004) days, compared to 544 (IQR: 254–965) days in the isolated LVAD group (P=0.187). Their status at 1-year is 57% alive on the device, 30% deceased and 13% have been successfully transplanted.
Thirty-day mortality is comparable between those with and without concomitant surgery (4% vs. 6%, P=0.426). The in-hospital mortality is significantly higher in the group with additional cardiac procedures (22% vs. 14%, P=0.05) but the one-year survival is similar (71% vs. 79%, P=0.106). See also Figure 1.
When comparing aortic and tricuspid valve procedures, the two most common procedures, we found a greater amount of DT patients (44% vs. 16%; P=0.035) with a significantly higher age {median age 65 [57–71] vs. 57 [50–65] years, P=0.019} in the aortic valve group. All other baseline characteristics were similar. Furthermore, no significant differences in terms of survival [30-day mortality aortic valve replacement (AVR) 12% vs. 2% TVR, P=0.137; in-hospital mortality 25% AVR vs. 22% TVR, P=0.743; one-year survival 56% AVR vs. 77% TVR, P=0.202] or need for RV-support (13% AVR vs. 37% TVR, P=0.063) were detected.
Discussion
Valvular pathologies are a common finding in terminal heart failure and concomitant cardiac surgery at the time of LVAD implantation, especially tricuspid, aortic or mitral valve procedures, is frequent, providing reasonable short- and mid-term results.
We analysed our LVAD recipients undergoing additional cardiac surgery and compared them to isolated LVAD implantations. As a main finding of the study, we observed a significantly higher in-hospital mortality and a tendency towards a lower 1-year survival for patients having combined procedures. Furthermore, there was a significantly higher need for temporary RV support.
The study cohort is rather heterogenous in terms of some baseline characteristics, including reason for and duration of heart failure or INTERMACS level. Nevertheless, our results add to what has been published by other groups and registers.
Guidelines are clear regarding the necessity to address more than mild aortic regurgitation or intracardiac shunts (Class I indication according to current ISHLT guidelines) in atrioventricular valve procedures, but this controversial in the literature. A recent INTERMACS database analysis revealed that in tricuspid regurgitation, the repair had no survival benefit. On the contrary, they found a higher risk of bleeding, arrhythmia and stroke, and no improvement in quality of life. The main finding was that tricuspid valve procedure in patients for whom, tricuspid repair may be considered per current ISHLT recommendation, was associated with increased mortality (10). Veen and colleagues presented data from the EUROMACS registry, showing comparable survival rates for patients with or without concomitant tricuspid valve procedure at thirty days (13% vs. 10%), in-hospital (20% vs. 17%), and one year, but diverging survival curves after two years of follow up (76% in isolated LVADs vs. 63% in patients with concomitant procedures) (7). The effect of tricuspid repair seemed to be unsustainable, resulting in comparable risks for a recurrence of moderate to severe regurgitation after one-year.
Repair of mitral valve regurgitation has been shown to improve hemodynamic parameters (pulmonary hypertension, pulmonary vascular resistance) and hospital readmission rates after LVAD implantation. However, no survival benefit could be found (4,8,9). One-year survival here was comparable, with 72% vs. 60% (P=0.81), but still worse than what we achieved in our patients.
These results and those of several other studies imply beneficial effects of concomitant valve surgery in the acute phase, however long-term effects remain the subject of controversial discussion.
It was suggested that patients in need of valve repair are their own entity of LVAD recipients. Such patients are less acute with a longer history of heart failure and fewer ischemic etiologies (7). Valvular insufficiencies, especially of the tricuspid valve, are probably—in the absence of structural defects—more an expression of secondary long-term effects of dilatation and pulmonary hypertension on the RV function in chronic heart failure rather than a symptom to treat per se.
In our study we can confirm these findings. Isolated LVAD recipients presented with a significantly higher proportion of ischemic disease, were more often in INTERMACS level one, and in higher need for preoperative ECLS.
The second finding, the tendency of an increased need for temporary RV support, can at least partially be explained by the higher proportion of chronic heart failure patients. Unfortunately, the exact details of preoperative RV status cannot be drawn from the retrospective data set, but this observation is in line with what is reported by the aforementioned studies. Mullan et al. found a significantly higher need for temporary RV support in patients undergoing concomitant tricuspid valve procedures (4.4% vs. 3.3%, P=0.001), and Sugiura et al. also reported an early RV failure rate of 28% in LVAD recipients with concomitant valve procedures (10,12). Even though the difference was statistically not significant, it is still interesting that in our cohort, patients undergoing aortic valve procedures at the time of LVAD implantation had the highest percentage of short-term mortality (12% thirty-day mortality), whereas those undergoing tricuspid annuloplasty as concomitant procedure to LVAD implantation showed the highest need for temporary RV support (37%). This finding might be associated with the need for aortic cross-clamping in aortic valve surgery or slightly longer CPB times, leading to an increased inflammatory response which then negatively influences perioperative morbidity and mortality (16). Another factor explaining this finding might be the older age and greater portion of DT patients that was observed for these patients.
Overall, we can state that concomitant cardiac procedures in LVAD recipients remain controversial. It is debatable whether beneficial effects can be expected in the long run, since study results are highly diverse and often present opposing results, possibly caused by the heterogenous patient groups. There is evidence for improved hemodynamics after repair of mitral valve regurgitation, probably enhancing transplantability and improving late RV function. However, recent guidelines only suggest to address aortic valve regurgitation when more than mild and to close intracardiac shunts (Class I indication). In the absence of prospective randomized data resulting in reliable guidelines, performance of a concomitant cardiac surgery at the time of LVAD implantation remains mostly an individual choice.
Limitations
The present study is limited by its retrospective and non-randomized design. This has to be considered when evaluating the differences between patients with and without concomitant cardiac procedures.
Conclusions
Concomitant cardiac procedures in LVAD recipients are common but associated with an increased perioperative morbidity and mortality. The longer-term benefits of atrio-ventricular valve procedures remain unclear.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: DZ, DW and TS: Research Grants, Travel Grants, Advisors and Proctors for Abbott and Medtronic, all institutional Grants from Abbott, Medtronic, Edwards, Berlin Heart. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Brewer RJ, Cabrera R, El-Atrache M, et al. Relationship of tricuspid repair at the time of left ventricular assist device implantation and survival. Int J Artif Organs 2014;37:834-8. [Crossref] [PubMed]
- Stulak JM, Tchantchaleishvili V, Haglund NA, et al. Uncorrected pre-operative mitral valve regurgitation is not associated with adverse outcomes after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2015;34:718-23. [Crossref] [PubMed]
- Taghavi S, Hamad E, Wilson L, et al. Mitral valve repair at the time of continuous-flow left ventricular assist device implantation confers meaningful decrement in pulmonary vascular resistance. ASAIO J 2013;59:469-73. [Crossref] [PubMed]
- Aggarwal A, Raghuvir R, Eryazici P, et al. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. Ann Thorac Surg 2013;95:493-8. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- Veen KM, Caliskan K, de By TMMH, et al. Outcomes after tricuspid valve surgery concomitant with left ventricular assist device implantation in the EUROMACS registry: a propensity score matched analysis. Eur J Cardiothorac Surg 2019;56:1081-9. [Crossref] [PubMed]
- Imamura T, Nnanabu J, Rodgers D, et al. Hemodynamic effects of concomitant mitral valve surgery and left ventricular assist device implantation. ASAIO J 2020;66:355-61. [Crossref] [PubMed]
- Tanaka A, Kitahara H, Onsager D, et al. Impact of residual valve disease on survival after implantation of left ventricular assist devices. Ann Thorac Surg 2018;106:1789-96. [Crossref] [PubMed]
- Mullan C, Caraballo C, Ravindra NG, et al. Clinical impact of concomitant tricuspid valve procedures during left ventricular assist device implantation. J Heart Lung Transplant 2020;39:926-33. [Crossref] [PubMed]
- Holley CT, Fitzpatrick M, Roy SS, et al. Aortic insufficiency in continuous-flow left ventricular assist device support patients is common but does not impact long-term mortality. J Heart Lung Transplant 2017;36:91-6. [Crossref] [PubMed]
- Sugiura T, Kurihara C, Kawabori M, et al. Concomitant valve procedures in patients undergoing continuous-flow left ventricular assist device implantation: a single-center experience. J Thorac Cardiovasc Surg 2019;158:1083-9.e1. [Crossref] [PubMed]
- Haberl T, Riebandt J, Mahr S, et al. Viennese approach to minimize the invasiveness of ventricular assist device implantation. Eur J Cardiothorac Surg 2014;46:991-6; discussion 996. [Crossref] [PubMed]
- Riebandt J, Sandner S, Mahr S, et al. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann Thorac Surg 2013;96:1094-6. [Crossref] [PubMed]
- Riebandt J, Wiedemann D, Laufer G, et al. Sternotomy Sparing Thoratec HeartMate 3 Implantation Via Bilateral Minithoracotomy. Innovations (Phila) 2018;13:74-6. [Crossref] [PubMed]
- Whitten CW, Hill GE, Ivy R, et al. Does the duration of cardiopulmonary bypass or aortic cross-clamp, in the absence of blood and/or blood product administration, influence the IL-6 response to cardiac surgery? Anesth Analg 1998;86:28-33. [PubMed]







