Hemodynamic outcomes after valve-in-valve transcatheter aortic valve replacement: a single-center experience
Introduction
Redo aortic valve open surgery is associated with increased morbidity and mortality, owing to factors such as patient age and comorbidities, difficulty in surgical exposure and dissection, and surgical complexity (1,2). Recently, valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR) has emerged as an alternative to open surgery, with multicenter registries showing good clinical outcomes and acceptable safety profiles (3-5).
Hemodynamic outcomes are difficult to predict, given the variability in patient characteristics, but are known to be worse after ViV-TAVR than after native-valve TAVR or redo aortic valve surgery; one registry study found that up to 30% of patients had mean gradients >20 mmHg (4). Contributive factors include surgical valve size, surgical valve type, patient-prosthesis mismatch, and type of transcatheter valve (5-9). The relative contribution of each factor is not completely understood.
We conducted this study to better characterize hemodynamic outcomes after ViV-TAVR in a large single-center cohort across a variety of clinically relevant categories, including surgical valve type, size, and internal diameter. Given the evolving use of fracturing for ViV-TAVR, we included a focus on surgical valves that are not amenable to fracturing—specifically, the Trifecta (Abbott, Abbott Park, IL) and Hancock (Medtronic, Fridley, MN) aortic valves (10).
Methods
Patient population and data collection
In this retrospective study, we identified all patients in our institutional interventional database who underwent ViV-TAVR for a degenerated aortic valve bioprosthesis between January 1, 2013 and March 31, 2019. No patients were excluded.
Patients were defined as having significant surgical valve degeneration in accordance with 2009 American Society of Echocardiography guidelines (11). Stenotic valvular degeneration was defined as a mean gradient >40 mmHg, peak aortic jet velocity >4 m/s, and an effective orifice area <0.8 cm2. Where there was discordance between mean gradient and effective area, we relied on the dimensionless obstructive index (<0.25 indicates severe stenosis). Regurgitant failure was defined as meeting echocardiographic criteria for severe regurgitation (12). Those with both moderate stenosis and moderate regurgitation were labeled as having mixed modes of failure.
Patient charts were reviewed to extract clinical data, including age, sex, surgical risk score, baseline comorbidities, and New York Heart Association (NYHA) class. Risk scores included the European System for Cardiac Operative Risk Evaluation score (EuroSCORE) (13) and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) (14). Surgical valve characteristics included brand, year of aortic valve replacement, type of bioprosthesis (stentless versus stented), size, internal diameter, and mode of failure (stenotic, regurgitant, or mixed). Procedural variables included use of general anesthesia, transcatheter valve type and size, access site, procedure time, use of balloon dilatation, and use of valve fracturing.
Endpoints
For patients who were followed at our institution, the follow-up timeline is determined by the primary interventionalist. Typically, patient follow-up occurs 30 days after discharge (the discharge follow-up) and at one year (the outpatient follow-up) unless the patient opts to follow up with a primary cardiologist at an outside institution. Transthoracic echocardiography is performed at both visits.
Clinical endpoints included technical success, in-hospital adverse events, 30-day readmission, and 30-day all-cause mortality. Technical success was defined as correct anatomical positioning of the transcatheter valve in the absence of 30-day mortality. In-hospital adverse events included acute kidney injury, stroke, vascular complications, worsening congestive heart failure, new onset or worsening of atrial fibrillation, permanent pacemaker implantation, coronary obstruction, and respiratory failure. Acute kidney injury, stroke, vascular complications, worsening congestive heart failure, atrial fibrillation, and permanent pacemaker implantation were defined according to Valve Academic Research Consortium-2 (VARC-2) criteria (15). Respiratory failure was defined as: desaturation below 89%, with a new oxygen requirement of at least two liters after ViV-TAVR; hypercarbic respiratory failure with acute rise in partial pressure of carbon dioxide to >45 mmHg in a patient who is not a chronic retainer; failure to extubate within 24 hours; or need for reintubation during the index hospitalization.
Imaging endpoints of interest included mean and peak aortic valve gradients, aortic valve area, presence of paravalvular leak, and dimensionless obstructive index. These were obtained from transthoracic echocardiograms collected at baseline, after ViV-TAVR during the index hospitalization, and at the outpatient follow-up.
Procedural details
All decisions regarding patient candidacy for ViV-TAVR versus open surgery were determined during a multidisciplinary team meeting involving interventional cardiologists, cardiothoracic surgeons, advanced imagers, and diagnostic cardiologists.
Procedural planning was complex and necessitated the consideration of various factors. All patients underwent preoperative computed tomography angiography of the heart and peripheral vasculature to evaluate risk for coronary obstruction, degree of aortic valve and arch calcification, and size and caliber of the peripheral vasculature. Patients with large-enough peripheral vessels underwent a total percutaneous transfemoral approach; otherwise, a mini-thoracotomy was performed for apical delivery. Some early procedures required femoral cutdown.
The ViV-TAVR was performed in standard fashion, as described elsewhere (16,17). All procedures were performed under transesophageal echocardiographic and fluoroscopic guidance. The valve-in-valve application was used to determine transcatheter valve size on the basis of known surgical aortic valve characteristics. The choice of a self-expanding versus balloon-expandable valve was based on operator preference. Valves were positioned in standard fashion under rapid pacing, with position confirmed via fluoroscopy and transesophageal echocardiography before final deployment.
If invasive mean gradients obtained after valve deployment were elevated, dilatation was performed at the primary operator’s discretion. Valve fracturing, if employed, was carried out according to previously described methods (18-20). Whether valve fracturing was performed before or after transcatheter valve implantation was at the operator’s discretion.
Statistical analysis
Clinical, procedural, and imaging variables were reported as median with interquartile ranges or means with standard deviations, as appropriate. Outcomes were compared across variables of interest (including surgical valve type, size, and internal diameter) by using t-test and chi-square methods, as appropriate. For non-normally distributed data and small sample sizes, we used Wilcoxon rank sum and Fisher exact tests.
Alpha was set at P<0.05. All statistical analyses used R version 3.5.3 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline clinical characteristics
We identified 89 patients who underwent ViV-TAVR to repair a degenerated aortic valve bioprosthesis during the study period. Baseline clinical characteristics are shown in Table 1. Mean age was 69.0±12.6 years. Most patients were men (61%). Median STS-PROM score was 5.4 [3.2–8.5]. Baseline left ventricular ejection fraction (LVEF) was 55.0%±9.7%, with 11% of patients having an LVEF <40%. Three-quarters of the sample had NYHA functional class III or IV, 35% had a history of percutaneous coronary intervention, and 20% had a pre-existing permanent pacemaker.
Table 1
| Variable | Data |
|---|---|
| Male sex | 54 (61%) |
| Age, years | 69.0±12.6 |
| EuroSCORE | 9.7 [5.64–13.2] |
| STS-PROM score | 5.4 [3.2–8.5] |
| LVEF, % | 55.0±9.7 |
| LVEF <40% | 10 (11%) |
| Coronary artery disease | 60 (67%) |
| Percutaneous coronary intervention | 31 (35%) |
| Type 2 diabetes | 27 (30%) |
| Atrial fibrillation | 24 (27%) |
| Peripheral vascular disease | 31 (35%) |
| Chronic obstructive pulmonary disease | 33 (37%) |
| Permanent pacemaker implantation | 18 (20%) |
| NYHA class III or IV | 67 (75%) |
Data are expressed as n (%), mean ± standard deviation, or median [interquartile range]. EuroSCORE, European System for Cardiac Operative Risk Evaluation score; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality.
Procedural characteristics
Procedural details are provided in Tables 2,3. Most cases were performed via a total percutaneous route (81%) under general anesthesia (85%). The type of transcatheter valve was split almost evenly between self-expanding (47%) and balloon-expandable (53%). Balloon dilatation was performed in 16% of patients before valve deployment and in 29% after valve deployment. Valve fracturing was employed in five cases (6%).
Table 2
| Variable | Data |
|---|---|
| General anesthesia | 76 (85%) |
| Procedure time, min | 142 [98–180] |
| Fluoroscopy time, min | 30 [19–40] |
| Access site | |
| Total percutaneous | 72 (81%) |
| Femoral cutdown | 13 (15%) |
| Transapical | 2 (2%) |
| Subclavian | 1 (1%) |
| Axillary | 1 (1%) |
| Balloon dilatation | |
| Before ViV-TAVR | 14 (16%) |
| After ViV-TAVR | 26 (29%) |
| Valve fracture | 5 (6%) |
Data are expressed as n (%) or median [interquartile range]. ViV-TAVR, valve-in-valve transcatheter aortic valve replacement.
Table 3
| Variable | n (%) |
|---|---|
| Surgical valve characteristics | |
| Mode of failure | |
| Stenosis | 52 (58%) |
| Regurgitation | 21 (24%) |
| Mixed | 16 (18%) |
| Type | |
| Stented | 67 (75%) |
| Stentless | 22 (25%) |
| Internal diameter, mm | |
| ≤19 | 39 (45%) |
| >19 | 48 (55%) |
| Size, mm | |
| ≤21 | 33 (38%) |
| 23–25 | 42 (48%) |
| ≥27 | 12 (14%) |
| Surgical valve breakdown | |
| Carpentier-Edwards Perimount (Edwards Lifesciences Corp, Irvine, CA) | 6 (7%) |
| David procedure | 1 (1%) |
| Epic (Abbott) | 3 (3%) |
| Freestyle (Medtronic) | 5 (6%) |
| Hancock (Medtronic) | 1 (1%) |
| Homograft | 14 (16%) |
| Magna (Edwards Lifesciences Corp.) | 3 (3%) |
| Mitroflow (Sorin Group USA Inc, Arvada, CO) | 5 (6%) |
| Mosaic (Medtronic) | 21 (24%) |
| Perimount (Edwards Lifesciences Corp.) | 3 (3%) |
| Ross procedure | 1 (1%) |
| Toronto (St. Jude Medical, Minneapolis, MN) | 1 (1%) |
| Trifecta (Abbott) | 25 (28%) |
| Transcatheter aortic valve characteristics | |
| Type | |
| Balloon-expandable | 47 (53%) |
| Self-expanding | 42 (47%) |
| Size, mm | |
| 20 | 9 (10%) |
| 23 | 45 (51%) |
| 26 | 22 (25%) |
| 29 | 10 (11%) |
| 34 | 3 (3%) |
Most of the failed surgical bioprostheses were stented (75%). Of the 22 stentless bioprostheses, 14 were homografts. Valve failure was mostly stenotic (58%), with another 18% failing due to a combination of stenosis and regurgitation and 24% having purely regurgitant failure. Surgical valve sizes in this cohort were small; almost half (45%) of the bioprostheses had internal diameters ≤19 mm.
Stented bioprostheses tended to be smaller. Of 67 stented surgical valves, 32 (48%) had a valve size ≤21 mm, and 39 (58%) had an internal diameter ≤19 mm. In comparison, of 22 stentless bioprostheses, only one (5%) had a valve size ≤21 mm, and none had an internal diameter ≤19; valve size and internal diameter characteristics were not available for two of the stentless valves. Additionally, a greater proportion of the stented valves had a stenotic mode of failure [50/67 (75%), versus 7/22 (32%) for stentless].
Early clinical outcomes
Of the 89 procedures, 88 (99%) were technically successful. One patient’s transcatheter valve had to be surgically explanted during the index hospitalization due to persistently severely elevated aortic gradients. All patients survived to discharge.
In-hospital adverse events are listed in Table 4. Permanent pacemaker implantation was required in 4% of patients. Only one transient ischemic event and no coronary obstructions were noted. There were three minor vascular complications: one access site hematoma, one foreign device embolization that was successfully retrieved without clinical sequelae, and one iliac dissection that required stenting during the procedure.
Table 4
| Event | n (%) |
|---|---|
| 30-day mortality (n=74)* | 0 (0%) |
| 30-day readmission (n=74)* | 9 (12%) |
| Permanent pacemaker | 4 (4%) |
| Respiratory failure | 7 (8%) |
| Acute kidney injury | 3 (3%) |
| Transient ischemic attack | 1 (1%) |
| Congestive heart failure exacerbation | 5 (6%) |
| Atrial fibrillation | 6 (7%) |
| Coronary obstruction | 0 (0%) |
| Minor vascular complication | 3 (3%) |
*, after discharge, 15 of the 89 original patients were lost to follow-up or did not otherwise provide follow-up records.
After discharge, 15 patients (17%) were lost to follow-up or otherwise did not have records available after discharge. Of the 74 patients for whom discharge follow-up data were available, nine (12%) were readmitted within 30 days of discharge, and none died.
Overall hemodynamic results
Outpatient follow-up transthoracic echocardiograms were available for 65% of patients. The median time to outpatient follow-up transthoracic echocardiography was 331 [67–394] days.
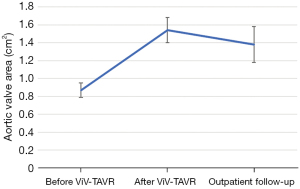
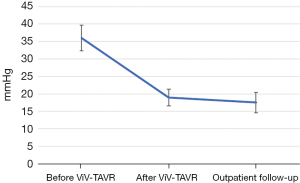
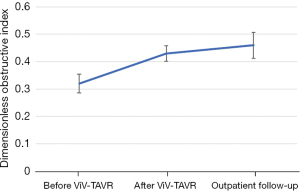
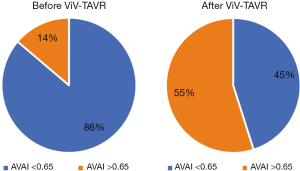
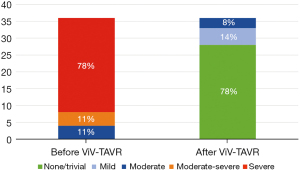
The baseline aortic valve area (AVA) of 0.87±0.31 cm2 improved to 1.54±0.54 cm2 (P<0.001) after ViV-TAVR. This improvement was maintained at the outpatient follow-up (1.38±0.55 cm2; P=0.80) (Figure 1). Mean gradients also improved significantly from baseline to post-procedure (36±18 versus 19±11 mmHg, P<0.001). This improvement also was maintained through outpatient follow-up (18±11 mmHg, P=0.48) (Figure 2). Similarly, the dimensionless obstructive index was improved post-procedure and maintained this trend at outpatient follow-up (Figure 3). Before ViV-TAVR, 86% of patients had severe patient-prosthesis mismatch; this percentage decreased to 45% after ViV-TAVR (P<0.001) (Figure 4). Of the 36 patients with at least moderate regurgitation at baseline, only three had at least moderate regurgitation after ViV-TAVR (Figure 5).
Hemodynamic gradients and valve sizes at baseline and after ViV-TAVR were stratified across several variables of interest, including surgical valve type (stented versus stentless), surgical valve internal diameter (≤19 versus >19 mm), transcatheter valve type (self-expanding versus balloon-expandable), and whether the surgical valve was Trifecta. Analyses were performed for the entire cohort and then repeated without the 21 patients who had purely regurgitant bioprosthesis failure (Table 5).
Table 5
| Variable | Count | Before ViV-TAVR | After ViV-TAVR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean |
P value | Mean gradient, mmHg | P value | DOI | P value* | AVA, cm2 | P value | Severe patient- |
P value | ||||
| Entire patient population (n=89) | |||||||||||||
| Internal diameter† | |||||||||||||
| ≤19 mm | 39 | 42±16 | 0.007* | 24±13 | 0.002* | 0.39±0.11 | 0.04* | 1.40±0.47 | 0.12 | 42% | 0.78 | ||
| >19 mm | 48 | 32±17 | 16±8 | 0.45±0.13 | 1.60±0.52 | 35% | |||||||
| SAVR type | |||||||||||||
| Stented | 67 | 39±16 | 0.009* | 22±11 | <0.001* | 0.41±0.12 | 0.02* | 1.47±0.47 | 0.18 | 40% | 0.75 | ||
| Stentless | 22 | 25±20 | 12±6 | 0.49±0.14 | 1.73±0.69 | 31% | |||||||
| ViV-TAVR type | |||||||||||||
| Balloon-expandable | 49 | 36±16 | 0.90 | 20±10 | 0.41 | 0.41±0.11 | 0.08 | 1.51±0.59 | 0.62 | 47% | 0.20 | ||
| Self-expanding | 40 | 36±20 | 19±12 | 0.45±0.14 | 1.58±0.49 | 26% | |||||||
| Fracturable valves | |||||||||||||
| Trifecta or Hancock | 26 | 38±18 | 0.53 | 20±12 | 0.70 | 0.40±0.12 | 0.18 | 1.43±0.45 | 0.35 | 38% | 0.99 | ||
| Other | 64 | 35±17 | 19±11 | 0.44±0.13 | 1.56±0.58 | 38% | |||||||
| Excluding 21 patients with purely regurgitant failure (n=68) | |||||||||||||
| Internal diameter | |||||||||||||
| ≤19 mm | 34 | 46±14 | 0.02* | 27±12 | 0.006* | 0.38±0.10 | 0.30 | 1.38±0.46 | 0.22 | 46% | >0.99 | ||
| >19 mm | 34 | 38±15 | 19±7 | 0.41±0.11 | 1.57±0.58 | 36% | |||||||
| SAVR type | |||||||||||||
| Stented | 58 | 42±13 | 0.19 | 23±11 | 0.005* | 0.39±0.10 | 0.25 | 1.44±0.46 | 0.55 | 42% | >0.99 | ||
| Stentless | 10 | 38±21 | 15±5 | 0.45±0.15 | 1.63±0.83 | 38% | |||||||
| ViV-TAVR type | |||||||||||||
| Balloon-expandable | 40 | 39±14 | 0.10 | 22±10 | 0.65 | 0.40±0.11 | 0.75 | 1.50±0.63 | 0.44 | 46% | 0.47 | ||
| Self-expanding | 28 | 45±15 | 23±12 | 0.39±0.11 | 1.39±0.35 | 32% | |||||||
| Fracturable valves | |||||||||||||
| Trifecta & Hancock | 20 | 44±15.1 | 0.40 | 22.7±11.7 | 0.80 | 0.38±0.09 | 0.27 | 1.42±0.43 | 0.70 | 39% | >0.99 | ||
| Other | 48 | 40.6±14.4 | 21.9±10.2 | 0.41±0.11 | 1.48±0.58 | 41% | |||||||
*, indicates significant at P<0.05; †, internal diameter characteristics were not available for 2 valves. AVA, aortic valve area; DOI, dimensionless obstructive index; SAVR, surgical aortic valve replacement; ViV-TAVR, valve-in-valve transcatheter aortic valve replacement.
Post-procedural transvalvular gradients >20 mmHg were seen in 49% of patients whose bioprosthesis had an internal diameter ≤19 mm and in 23% of those whose bioprosthesis had an internal diameter >19 mm. After ViV-TAVR, valves with internal diameters ≤19 mm had higher mean hemodynamic gradients, compared with larger valves (24±13 versus 16±8 mmHg, P=0.002) (Table 5). This difference remained significant when valves with purely regurgitant modes of failure were excluded (27±12 versus 19±7 mmHg, P=0.006). Stented surgical valves also had higher hemodynamic gradients than stentless surgical valves (22±11 versus 12±6 mmHg, P<0.001). This difference remained significant when valves with purely regurgitant modes of failure were excluded (23±11 versus 15±5 mmHg, P=0.005).
No statistical differences were observed between self-expanding and balloon-expandable valves in terms of AVA, mean gradients, or patient prosthesis mismatch. However, the frequency of patient-prosthesis mismatch with balloon-expandable valves was almost double that with self-expanding valves (47% versus 26%, P=0.2), although the difference was not statistically significant. A similar trend was seen for surgical valves with internal diameters ≤19 mm versus larger valves (60% versus 31%, P=0.23) (data not shown).
Trifecta or Hancock surgical valves were found in 26 patients. Mean gradients for these patients were 38±18 mmHg before ViV-TAVR and 20±12 mmHg after ViV-TAVR. A considerable percentage of patients (36%) had mean gradients >20 mmHg after ViV-TAVR. Trifecta valves were not statistically different from other surgical valves in terms of aortic pressure gradients, AVAs, and rates of patient-prosthesis mismatch.
Discussion
Data supporting the safety and efficacy of ViV-TAVR is rapidly accumulating, with various multicenter registries showing the procedure to be a safe and effective alternative to repeat open surgery in eligible patients (3,5). However, hemodynamic outcomes for ViV-TAVR are inferior to those for TAVR in native aortic valves (3,9,21,22). Up to 30% of ViV-TAVR patients have post-procedural transvalvular gradients >20 mmHg, which according to the updated VARC-2 criteria constitutes procedural failure (15). In this report, we aimed to describe our single-center hemodynamic and other outcomes after ViV-TAVR across various factors of interest.
Small internal diameters
Surgical bioprostheses with small internal diameters (≤19 mm) had significantly worse transvalvular gradients after ViV-TAVR than bioprostheses with internal diameters >19 mm. Within our sample, 49% of patients whose bioprosthesis had an internal diameter ≤19 mm had post-procedural transvalvular gradients >20 mmHg (and therefore hemodynamic failure, according to VARC-215), compared with only 23% of those with larger bioprostheses. Small surgical valves being associated with worse hemodynamic outcomes is well known (3,6,23,24). The notable result from our cohort is that rates of hemodynamic failure approached 50%, significantly higher than what has been previously described.
Patients with elevated hemodynamic gradients may have worse clinical outcomes. Therefore, caution should be exercised when operating on small surgical valves, and alternative solutions should be considered. For operable candidates, surgery that includes aortic root enlargement is associated with acceptable gradients (25,26). For non-operable candidates, valve fracturing may be useful. This was employed in only five of our patients, limiting our ability to extract data about its efficacy.
Stented versus stentless surgical valves
Much less is known about ViV-TAVR for stentless valves, as they are considerably rarer than stented valves, accounting for about 10% of implanted bioprostheses (27). Typically, stentless valves are placed in patients with small aortic annuli in an effort to optimize hemodynamics and avoid patient-prosthesis mismatch. Because they lack a stent frame, stentless valves do not provide fluoroscopic landmarks and thus pose important challenges with regard to transcatheter valve landing and sizing (7,28-32). Duncan et al. (30) compared clinical outcomes between stentless versus stented valves in an international registry and found that patients with stentless valves had a higher rate of periprocedural adverse events (including device migration) than patients with stentless valves, but that both groups had similar 30-day and 1-year clinical outcomes overall.
In our study, stentless valves were associated with significantly better post-procedural gradients compared with stented valves. This was partly related to a higher rate of regurgitant degeneration among the stentless valves. The significant difference in aortic mean gradients remained even after including only stenotic modes of failure, although our sample sizes were very small. A possible explanation would be that the lack of a stent frame allows for better compliance and more complete transcatheter valve expansion. It is important to note that all stentless valves are not equivalent. Our stentless cohort included a substantial percentage of homografts (14/22). Whether outcomes among stentless subcategories differ warrants further study.
Trifecta and Hancock surgical valves
The advent of balloon fracturing has important implications for ViV-TAVR procedures, possibly allowing for better hemodynamic results in patients with small, rigid surgical aortic valves (10,33). An important limitation of valve fracturing is that it cannot be employed with all valves. Trifecta and Hancock are notable surgical bioprostheses that cannot be fractured, due to their design (10,34). Our cohort included 25 patients with Trifecta valves and one with a Hancock valve. These valves did not differ from the other valves with regards to mean gradient, aortic valve area, or patient-prosthesis mismatch.
Valve fracturing was seldom used within our cohort. Had it been used more frequently, a difference in hemodynamics might have been observed. Nevertheless, it is reassuring that postprocedural hemodynamics in patients with Trifecta valves were acceptable, with mean gradients comparable to what has been described in the literature. More than a third of these patients (36%) had mean gradients >20 mmHg after ViV-TAVR, and 33% had severe patient-prosthesis mismatch, but these findings are less likely to be related to Trifecta valves specifically than to limitations of the general cohort.
Balloon-expandable versus self-expanding transcatheter valves
Early in vitro experiments suggest that self-expanding valves may have better hemodynamic performance than balloon-expandable valves, owing to their supra-annular position (34,35). Thus, some operators prefer to implant self-expanding valves into small surgical aortic valves. In general, we found no statistical differences in hemodynamic outcomes between self-expanding and balloon-expandable valves. There was, however, a trend toward more frequent patient-prosthesis mismatch in patients with balloon-expandable valves versus self-expanding valves. That trend persisted, although not significantly, when surgical aortic valves were compared by inner diameter [≤19 mm (60%) versus >19 mm (31%)]. This trend should be investigated in larger cohorts.
Whether or not severe patient-prosthesis mismatch has a bearing on clinical outcomes remains debatable. One-year outcomes based on data from various ViV-TAVR registries do not show an impact on 1-year mortality (36). The correlation between severe prosthesis mismatch and symptoms, readmissions, valve durability, and long-term outcomes is not well studied.
Limitations
Our study has several limitations. Because this was a single-center, unrandomized cohort, the study was prone to selection bias and random error. Our patient population was not powered for many of the clinical and hemodynamic endpoints evaluated. This issue was further compounded by our breaking the sample into smaller subgroups for comparison purposes, such that we could not control for relevant covariates (e.g., the interplay between surgical valve type and internal diameter). Thus, all findings should be considered exploratory and hypothesis generating. Also, our sample would be difficult to generalize, given its makeup: We had a high percentage of Trifecta valves and homografts, and a significant proportion of our patients had very small surgical aortic valves. Furthermore, long-term follow-up was lacking.
Conclusions
We present the hemodynamic outcomes of ViV-TAVR in a large single-center cohort, with multiple sub-analyses according to procedural characteristics. Stentless valves were associated with better pressure gradients relative to stented valves. Larger studies should be conducted to explore these results. These considerations should inform surgeons at the time of primary surgical aortic valve repair and can assist interventionalists in predicting outcomes and assessing patient candidacy for ViV-TAVR.
Acknowledgments
Jeanie F. Woodruff, BS, ELS, contributed to the editing of the manuscript.
Funding: None.
Footnote
Conflicts of Interest: Dr. Preventza participates in clinical trials with and/or consults for Terumo Aortic and W.L. Gore & Associates. Dr. Coselli participates in clinical studies with and/or consults for Terumo Aortic, Medtronic, W.L. Gore & Associates, CytoSorbents, and Abbott Laboratories and receives royalties and grant support from Terumo. None of the other authors have any potential conflict of interest with regard to the work described in this manuscript. This work was not funded by a grant or any other source of external funding.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Balsam LB, Grossi EA, Greenhouse DG, et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg 2010;90:1195-200; discussion 1201. [Crossref] [PubMed]
- Bortolotti U, Milano A, Mossuto E, et al. The risk of reoperation in patients with bioprosthetic valves. J Card Surg 1991;6:638-43. [Crossref] [PubMed]
- Deeb GM, Chetcuti SJ, Reardon MJ, et al. 1-Year Results in Patients Undergoing Transcatheter Aortic Valve Replacement With Failed Surgical Bioprostheses. JACC Cardiovasc Interv 2017;10:1034-44. [Crossref] [PubMed]
- Tuzcu EM, Kapadia SR, Vemulapalli S, et al. Transcatheter Aortic Valve Replacement of Failed Surgically Implanted Bioprostheses: The STS/ACC Registry. J Am Coll Cardiol 2018;72:370-82. [Crossref] [PubMed]
- Webb JG, Mack MJ, White JM, et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol 2017;69:2253-62. [Crossref] [PubMed]
- Kuehnel RU, Hartrumpf M, Erb M, et al. Hemodynamic Performance of Endovascular Valves as Valve-in-Valve in Small Stented Bioprosthesis. Thorac Cardiovasc Surg 2017;65:225-30. [PubMed]
- Murdoch DJ, Webb JG. Transcatheter valve-in-valve implantation for degenerated surgical bioprostheses. J Thorac Dis 2018;10:S3573-7. [Crossref] [PubMed]
- Patel JS, Krishnaswamy A, White J, et al. Optimizing hemodynamics of transcatheter aortic valve-in-valve implantation in 19-mm surgical aortic prostheses. Catheter Cardiovasc Interv 2018;92:550-4. [Crossref] [PubMed]
- Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J Am Coll Cardiol 2017;70:1013-28. [Crossref] [PubMed]
- Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv 2018;11:677-89. [Crossref] [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23; quiz 101-2. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9-13. [Crossref] [PubMed]
- O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg 2009;88:S23-42. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- Webb JG, Dvir D. Transcatheter aortic valve replacement for bioprosthetic aortic valve failure: the valve-in-valve procedure. Circulation 2013;127:2542-50. [Crossref] [PubMed]
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848-57. [Crossref] [PubMed]
- Allen KB, Chhatriwalla AK, Cohen DJ, et al. Bioprosthetic Valve Fracture to Facilitate Transcatheter Valve-in-Valve Implantation. Ann Thorac Surg 2017;104:1501-8. [Crossref] [PubMed]
- Sathananthan J, Sellers S, Barlow AM, et al. Valve-in-Valve Transcatheter Aortic Valve Replacement and Bioprosthetic Valve Fracture Comparing Different Transcatheter Heart Valve Designs: An Ex Vivo Bench Study. JACC Cardiovasc Interv 2019;12:65-75. [Crossref] [PubMed]
- Saygılı A, Yalçınbaş Y, Arnaz A, et al. Successful stenting of systemic venous pathway stenosis after double switch repair for congenitally corrected transposition of great arteries in a child. Turk Kardiyol Dern Ars 2014;42:571-3. [Crossref] [PubMed]
- Barbanti M, Costa G, Zappulla P, et al. Incidence of Long-Term Structural Valve Dysfunction and Bioprosthetic Valve Failure After Transcatheter Aortic Valve Replacement. J Am Heart Assoc 2018;7:e008440 [Crossref] [PubMed]
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [Crossref] [PubMed]
- Pibarot P, Simonato M, Barbanti M, et al. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC Cardiovasc Interv 2018;11:133-41. [Crossref] [PubMed]
- Scholtz S, Piper C, Horstkotte D, et al. Valve-in-valve transcatheter aortic valve implantation with CoreValve/Evolut R© for degenerated small versus bigger bioprostheses. J Interv Cardiol 2018;31:384-90. [Crossref] [PubMed]
- Kaneko T, Vassileva CM, Englum B, et al. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann Thorac Surg 2015;100:1298-304; discussion 1304. [Crossref] [PubMed]
- Stulak JM, Tchantchaleishvili V, Daly RC, et al. Conventional redo biological valve replacement over 20 years: Surgical benchmarks should guide patient selection for transcatheter valve-in-valve therapy. J Thorac Cardiovasc Surg 2018;156:1380-1390.e1. [Crossref] [PubMed]
- Gulbins H, Reichenspurner H. Which patients benefit from stentless aortic valve replacement? Ann Thorac Surg 2009;88:2061-8. [Crossref] [PubMed]
- Bapat V, Davies W, Attia R, et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: Technical considerations and results. J Thorac Cardiovasc Surg 2014;148:917-22; discussion 922-4. [Crossref] [PubMed]
- Duncan A, Davies S, Di Mario C, et al. Valve-in-valve transcatheter aortic valve implantation for failing surgical aortic stentless bioprosthetic valves: A single-center experience. J Thorac Cardiovasc Surg 2015;150:91-8. [Crossref] [PubMed]
- Duncan A, Moat N, Simonato M, et al. Outcomes Following Transcatheter Aortic Valve Replacement for Degenerative Stentless Versus Stented Bioprostheses. JACC Cardiovasc Interv 2019;12:1256-63. [Crossref] [PubMed]
- Huczek Z, Grodecki K, Scisło P, et al. Transcatheter aortic valve-in-valve implantation in failed stentless bioprostheses. J Interv Cardiol 2018;31:861-9. [Crossref] [PubMed]
- Sang SLW, Beute T, Heiser J, et al. Early Outcomes for Valve-in-valve Transcatheter Aortic Valve Replacement in Degenerative Freestyle Bioprostheses. Semin Thorac Cardiovasc Surg 2018;30:262-8. [Crossref] [PubMed]
- Chhatriwalla AK, Allen KB, Saxon JT, et al. Bioprosthetic Valve Fracture Improves the Hemodynamic Results of Valve-in-Valve Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv 2017;10:e005216 [Crossref] [PubMed]
- Reul RM, Ramchandani MK, Reardon MJ. Transcatheter Aortic Valve-in-Valve Procedure in Patients with Bioprosthetic Structural Valve Deterioration. Methodist Debakey Cardiovasc J 2017;13:132-41. [Crossref] [PubMed]
- Sedaghat A, Sinning JM, Utzenrath M, et al. Hydrodynamic Performance of the Medtronic CoreValve and the Edwards SAPIEN XT Transcatheter Heart Valve in Surgical Bioprostheses: An In Vitro Valve-in-Valve Model. Ann Thorac Surg 2016;101:118-24. [Crossref] [PubMed]
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014;312:162-70. [Crossref] [PubMed]






