Consensus on hypothermia in aortic arch surgery
Introduction
Cooling the brain down to hypothermic temperatures is sufficient to reduce brain metabolic requirements to such an extent that blood flow can be completely interrupted. As such, hypothermic circulatory arrest (HCA), as popularised by R.B. Griepp in the mid-1970s, is now considered an essential component of aortic arch surgery. Most notably, HCA offers surgeons a bloodless operating field and extended surgical time limit while meeting the body’s high metabolic demands. Although voluminous studies have demonstrated benefits of HCA over the past three decades, pioneers in cardiac surgery continue to push the boundaries and seek to improve this procedure. In conjunction with the recent increase in the implementation of selective antegrade cerebral perfusion during HCA, a paradigm shift has been the use of warmer hypothermic temperatures, which is argued to reduce re-warming time, minimize the degree of coagulopathy and improve survival outcomes.
In light of this transition, it is vital to assess how temperature impacts upon operative outcomes. An obstacle to this challenging task has been the lack of standardized nomenclature for various categories of hypothermia. Terms such as ‘deep hypothermic circulatory arrest’ and ‘moderate hypothermic circulatory arrest’ are currently used without any clear consistency. A consensus must be reached in order to facilitate better reporting, which will occasion a more thorough understanding of HCA, with the aim of developing more effective surgical procedures and patient selection guidelines. Recommendations on operative details should also be provided to guide good clinical practice.
An international panel of experts, representing high-volume aortic centers from Australia, Asia, Europe, and North America, have collaborated and reached the following consensus. Discrepancies in opinions were resolved by online voting system. Consensus was reached when greater than 50% of the panel concurred on a response. The first part of this report will address the consensus on hypothermia classifications, while the following portions will detail hypothermia induction and monitoring.
Consensus on hypothermia classification
The following categories have been proposed to classify various temperature ranges of systemic HCA (Table 1). This proposal is founded on the belief that brain metabolism is the key determinant of successful arrest temperature, and that other visceral organs can tolerate ischemic damage better (1). Furthermore, the following interpretations are borne on the evidence of human studies only, as these are perhaps more representative than the numerous existing animal studies.
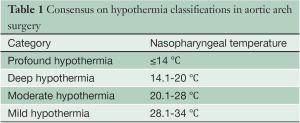
Full table
It is noted that while these classifications may seem like semantics, like much of medicine and surgery, categorising and standardization is the first step towards better understanding of the topic. This classification is intended solely to establish a standardized reporting system, and should not be viewed as a recommendation for changes in surgical practice.
Profound hypothermia: ≤14 °C
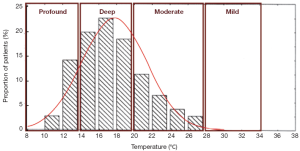
Profound hypothermia is defined as less than or equal to 14 °C. At 14 °C, in a study of 47 patients undergoing circulatory arrest for aortic surgery, approximately 14% of patients have not achieved electrocerebral silence (ECS) (Figure 1) (2), while a larger study of 325 patients found 22% of patients did not achieve ECS at this temperature (3). Profound HCA at 14 °C is also reported to provide at least 30-40 minutes of safe HCA time (4,5). Furthermore, between 10-15 °C, McCullough and colleagues have shown that there is only a 5% increase in cerebral metabolic rate (from 11% to 16%) (4).
As such, we feel this arrest temperature is not frequently used because it may not confer much physiological cerebral advantage, particularly when it is weighed against the increased time required to cool and rewarm the patient and greater potential for coagulation derangements, as brain activity has already ceased in the vast majority of patients, and there is only a small decrease in cerebral metabolic rate.
Deep hypothermia: 14.1-20 °C
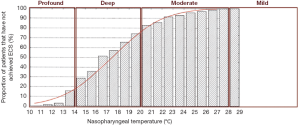
Deep hypothermia is defined as between 14.1-20 °C. At 20 °C, 75-98% of patients have not achieved ECS, while below 14.1 °C, 14-22% of patients have not achieved ECS (Figure 2) (2,3). Deep HCA between 14.1-20 °C also affords approximately 20-30 minutes of safe HCA time (Figure 3) (4), sufficient for open-distal, hemi-arch repairs.
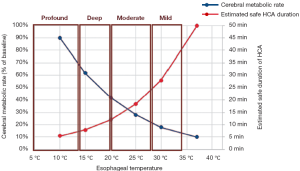
This is the most common range of temperatures reported for deep HCA in the literature. Additionally, between 15-20 °C, cerebral metabolic rate increases by 8%, with accelerating rates of increase beyond this range (4). HCA at 15 °C was also not associated with increased postoperative haemorrhage or early pulmonary or renal dysfunction compared to HCA at 28 °C, although this may be have been influenced by pre-emptive supplementation of blood products (6). We believe that this temperature range is perhaps the most therapeutically beneficial in minimising brain trauma, based on the above evidence.
Moderate hypothermia: 20.1-28 °C
Moderate hypothermia is defined as between 20.1-28 °C. At 28 °C, 99-100% of patients have not achieved ECS, while at 20.1 °C, 75-98% of patients have not achieved ECS (2,3). Moderate HCA between 20.1-28 °C only affords approximately 10-20 minutes of safe HCA time (4).
At 21 °C, hippocampal neurons have been shown to survive for 15 hours in anoxic conditions, significantly more than the 5 hours afforded at 28 °C. In the absence of glucose and oxygen, cooling to 21 °C confers a 300% increase in neuronal survival time compared to 28 °C (7). When the temperature increases from 20 to 25 °C, brain activity will also increase to 37% (a 13% increase, compared to only a 8% increase in the previous 5 °C increment) (4) (Figure 3).
Furthermore, within this category, Kamiya and colleagues did not find any significant differences in mortality and morbidity for lower body circulatory arrest between 20-24.9 °C and 25-28 °C propensity-matched cohorts, although there was a trend for lower post-operative inflammatory response and reduced re-exploration for bleeding in the warmer group (1).
Taken together, this suggests that arrest temperatures higher than 28 °C have the potential to impair the awakening brain, as it is becoming metabolic active at an accelerating rate.
Mild hypothermia: 28.1-34°C
This category offers only short protective effect on organs, with less than 10 minutes of safe HCA time (4). Above 28 °C, Stecker et al. estimated that nearly 100% of patients will not achieve ECS; these results are confirmed by James and colleagues, who found that none of their 325 patients could attain ECS for temperatures greater than 25 °C (2,3). This temperature range offers less than 10 minutes of safe HCA time (4).
Clinical recommendations
Induction of hypothermia
Institutional preferences have favoured a variety of routes for cardiopulmonary bypass arterial inflows over the years, including the aorta, and the femoral, iliac, axillary, innominate, carotid, and subclavian arteries. While the debate between axillary versus femoral cannulation is not the focus of this statement, it worthwhile highlighting the following facts.
Cannulation of the axillary artery provides several benefits, including (8-10):
- Ability for later application of antegrade cerebral perfusion;
- Eliminating risk of retrograde embolization from the descending aorta;
- Reducing chances of malperfusion and hypoperfusion;
- Reducing risk of retrograde dissection;
- Potentially redirecting flow into the true lumen in dissections, and decompressing the expanded false lumen;
- Artery is usually free from atherosclerotic plaques.
It has been recommended that axillary cannulation be performed via an anastomosed graft, instead of direct cannulation, to minimise issues related to small axillary arteries, high resistance from using cannula with side openings, and possible risk of compartment syndrome (10,11).
While femoral artery cannulation is a technique that has been preferred historically, and can be performed rapidly, this route is associated with higher incidence of post-operative embolic stroke, particularly due to retrograde flow through the potentially diseased aorta. Malperfusion may also occur as a result of unpredictable shifting of intimal flaps in acute dissections (8).
It must be acknowledged that there is a lack of conclusive evidence that clearly endorses axillary over femoral cannulation and some of the superior results from axillary cannulation may be related to maintenance of continuous antegrade cerebral perfusion (12). Clinical decision on axillary versus femoral cannulation is ultimately dictated by patient factors and surgeon preference.
Monitoring of hypothermia
Several sites have been employed to measure body temperature, including the brain, tympanum, nasopharynx, esophagus, bladder, and rectum. As the success of hypothermia is contingent upon elimination of cerebral metabolism, it is the brain temperature that should be measured. However, capturing true brain temperatures is “difficult”, so suitable substitute sites must be used.
A study in 1995 examined the accuracy of various temperature monitoring sites as compared to a brain probe inserted into the cerebral cortex (13). Temperatures at various stages of the operation were recorded until HCA occurred at brain temperature of 16.7 °C, after which patients were rewarmed following cerebral aneurysmal repair. Of the 6 instances when temperatures were recorded (cerebral cortical temperatures of 32.6, 23.4, 16.7, 17.5, 27.4 and 35.8 °C), the greatest disparity was seen with perfusate temperature, which had a mean difference of 3.1 °C as compared to the brain. The least difference was measured at the distal esophagus, which had only a 0.5 °C mean difference compared to brain temperature (see Figure 4). Furthermore, in a separate study, temperatures measured in the nasopharynx and in the proximal esophagus (at 24 cm) provided the closest approximations to brain temperature (both sites had a mean difference of 0.4 °C) (14).
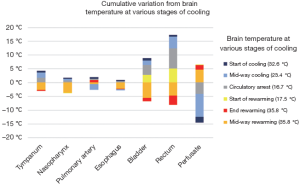
However, the above studies were conducted in patients who had predominantly closed-chest operations. Arch surgery necessitates the opening of chest, which increases temperature loss at the lower esophageal sites. As the nasopharynx provides comparable measurements [only 0.8 °C mean difference (13)], and by virtue of its close anatomical proximity to the brain (the nasopharynx is supplied by the branches of the external carotid artery, which is perfused during selective antegrade cerebral perfusion), it is well suited for use as the site of temperature measurement in lieu of invasive brain measurements. However, warmer temperatures may affect the accuracy of nasopharyngeal temperature serving as an indicator of brain temperature.
The bladder is often used as a site to measure core temperature, as it is more easily accessible and is not affected by temperature of adjunct cerebral perfusate. However, bladder temperature often lags substantially behind nasopharyngeal temperatures (and thus is slightly warmer during the cooling phase, and significantly cooler during the warming phase). Like rectal temperature, bladder temperature is not well suited for monitoring during cases necessitating HCA. If bladder temperature is used, it should be used in conjunction with a more cranial site and care should be taken to correlate the temperatures.
Conclusions
The rate at which adjunctive surgical techniques and variations of existing practices used in aortic surgeries have evolved has significantly outpaced our thorough understanding of these changes. To date, despite the numerous institutional reports on hypothermic circulatory arrest, little has been done to standardise such reports, particularly for the categories of hypothermia. We have proposed four temperature ranges for hypothermia, based on EEG and metabolism studies of the brain. It is hoped that by standardizing this nomenclature and associated operative details, the impact of HCA on patient outcomes can be evaluated in a more consistent manner.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9.
- Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 2001;71:14-21.
- Andersen ND, James ML, Swaminathan M, et al. Predictors of electrocerebral inactivity with deep hypothermia. J Thorac Cardiovasc Surg 2013. [Epub ahead of print].
- McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999;67:1895-9; discussion 1919-21.
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31.
- Harrington DK, Lilley JP, Rooney SJ, et al. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann Thorac Surg 2004;78:596-601.
- Okada Y, Tanimoto M, Yoneda K. The protective effect of hypothermia on reversibility in the neuronal function of the hippocampal slice during long lasting anoxia. Neurosci Lett 1988;84:277-82.
- Coselli JS, LeMaire SA. Aortic Arch Surgery. West Sussex, UK: Blackwell Publishing Ltd, 2008.
- Moizumi Y, Motoyoshi N, Sakuma K, et al. Axillary artery cannulation improves operative results for acute type a aortic dissection. Ann Thorac Surg 2005;80:77-83.
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1274-84.
- Sinclair MC, Singer RL, Manley NJ, et al. Cannulation of the axillary artery for cardiopulmonary bypass: safeguards and pitfalls. Ann Thorac Surg 2003;75:931-4.
- Gulbins H, Pritisanac A, Ennker J. Axillary versus femoral cannulation for aortic surgery: enough evidence for a general recommendation? Ann Thorac Surg 2007;83:1219-24.
- Stone GJ, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology 1995;82:344-51.
- Whitby JD, Dunkin LJ. Cerebral, oesophageal and nasopharyngeal temperatures. Br J Anaesth 1971;43:673-6.





