Heart team approach for comprehensive management of aortic coarctation in the adult
Introduction
Coarctation of the aorta (COA) identified in the adult patient is a systemic condition, rather than simply an anatomic abnormality (1). The coarctation is usually a discreet narrowing at the aortic isthmus, but variations such as long-segment stenosis, arch involvement including hypoplastic arch and other locations of narrowing have been described. Bicuspid aortic valve, intercostal artery aneurysms, ventricular septal defects and intracranial artery aneurysms have also been associated with COA. COA comprises 5–10% of congenital cardiac anomalies, and most are diagnosed and treated early in life (2,3).
Some patients with post-ductal COA remain asymptomatic and escape detection until later in life, when systemic hypertension coupled with diminished femoral pulses or discordant upper extremity to lower extremity blood pressure measurements leads to further diagnostic evaluations (4). Re-coarctation at the site of prior repair presents in similar manner. Rarely, an adult with incidentally discovered COA will be normotensive at rest. Many of these, however, will have an exaggerated blood pressure response to exercise, and exercised induced hypertension (EIH) has been shown to correlate with cardiovascular risk—with up to 50% increased incidence of cardiovascular events (5). The sequelae of persistent systemic hypertension include increased afterload resulting in increased left ventricular (LV) wall mass, decreased compliance of arterial walls, blunted baroreceptor reflex and endothelial dysfunction. COA is associated with elastic fiber fragmentation, fibrosis, cystic medial necrosis of the aorta and increased intimal-medial thickness, even in infants and children, demonstrating the systemic nature of the condition (1). Congestive heart failure, intracranial aneurysm, post-coarctation aneurysm, dissection and post-repair complications, such as aneurysm, pseudoaneurysm and re-coarctation all contribute to a shortened lifespan. Untreated, the average age of death in patients with COA is 35 years, with 75% of patients deceased by 46 years of age (4,6). Despite successful treatment, as defined by post-repair reduction in pressure gradient to less than 10–20 mmHg, most patients will still require antihypertensive medications after repair (2,6,7). The goal for management of COA is early identification, early treatment, robust follow-up for post-repair complications, as well as continued hypertension management to mitigate the risks of long-standing, persistent hypertension. Moreover, reduction of LV mass will decrease congestive heart failure prevalence and improve long term survival (4,7).
Open repair of COA through a left thoracotomy was the predominant approach from the first reports in the 1940s until the last few decades (6,8,9). Depending on age and anatomy, repair is accomplished by end-to-end anastomosis, graft interposition, excision with patch angioplasty or extra-anatomic bypass. The first reports of endovascular interventions appeared in the 1980s with balloon angioplasty; however, due to elastic recoil and intimal hyperplasia, results were mixed. The use of uncovered and covered stent grafts for COA were initially described in the 1990s (10). With experience and improvement in devices, the majority of COA patients today are treated by endovascular methods. Over time, our center has increasingly used endovascular treatments for COA, which is now our predominant approach, especially in patients with recurrent stenosis following repair as a child (5,11). However, multiple generalized and patient-specific factors influence the choice of approach, and open treatment is offered to select patients. Therefore, we employ a multispecialty “heart team” involving cardiology, radiology, vascular surgery and cardiothoracic surgery to manage patients with COA at our center.
The purpose of this analysis is to review our multidisciplinary heart team approach to the management of COA based on our experience over the last decade with twenty-four adult patients. This includes a work-up algorithm, indications for intervention and selection of intervention, taking into account anatomic, physiologic and functional factors.
Methods
Study population and statistical analysis
We retrospectively reviewed twenty-four consecutive adult patients with COA managed at Baylor Scott & White The Heart Hospital from 2010 through 2020. Patient data was obtained via retrospective chart review. Patients were contacted at the time of analysis (April 2021) to ascertain current use or non-use of antihypertensive medications. Summary statistics using mean or count (percentage) are reported. The study was approved by the local institutional review board.
Preoperative evaluation
At presentation, patients have already been diagnosed with COA radiographically. We obtain upper and lower extremity (UE, LE) resting blood pressures in all patients. Our protocol is then to obtain computed tomography angiography (CTA) of the chest, abdomen and pelvis with 1.25 mm slice thickness. Cardiac gating is not necessary. We evaluate access vessels to ensure diameters are of adequate caliber to accommodate an endovascular delivery system and determine whether the femoral arteries have the appropriate anatomy for a percutaneous approach. We reconstruct the scan data into sagittal and coronal formats. A third party software is used to perform centerline reconstructions for accurate diameter and length measurements. Usually, these assessments are sufficient to plan and proceed. However, if the coarctation appears borderline and resting UE to LE peak systolic blood pressure gradient is less than 10 mmHg, we perform a diagnostic aortogram to verify the gradient across the lesion. Intravascular ultrasonography can also be employed for lesion evaluation and to assess the dynamic changes in aortic wall diameters during the cardiac cycle, information not obtainable by CTA alone.
In the absence of neurologic symptoms, we forego screening imaging of the brain. For any neurologic complaint, including persistent headaches, we obtain magnetic resonance angiography (MRA) or CTA of the brain and neck. Echocardiogram is not specifically recommended in the guidelines (7). However, because up to 75% of patients with COA have bicuspid aortic valve (BAV) and BAV is commonly associated with aortic stenosis (AS), aortic insufficiency (AI) and proximal aortic dilation, we do obtain transthoracic echocardiography (TTE) studies for all patients (7,12).
Indications for intervention
We recommend intervention for patients with hypertension and significant native or recurrent COA, defined as meeting at least one of the following criteria: (I) UE to LE mean Doppler systolic gradient or resting peak-to-peak systolic blood pressure gradient >20 mmHg; (II) UE to LE gradient >10 mmHg plus reduced LV systolic function, AI or collateral flow (7). If the patient is normotensive at rest, we obtain exercise stress gradients and apply the criteria above. If invasive pressure gradients are obtained, we utilize these values with the same thresholds (13). We also recommend intervention for anatomic severity, determined by the diameter ratio of the aortic isthmus to the aorta at the diaphragm hiatus (the Aortic Isthmic ratio), with a ratio <0.7 indicating significant disease warranting intervention. We also opt for intervention when there is evidence of increased LV mass on TTE, as this is a marker for significant physiologic derangement. Our justification for these recommendations is: (I) a 2020 from the Mayo Clinic demonstrated correlation of the aortic isthmus ratio with LV mass index, independent of physiologic variation (14); (II) all intervention decisions regarding our COA patients are made in a multidisciplinary team, which we feel limits bias and offers best medical advice; (III) guideline thresholds are typically designed for the point where the risks of intervening versus observing intersect, and we believe that our unbiased team approach and excellent results warrant more aggressive recommendations and (IV) we feel earlier intervention may limit some of the long-term sequelae of systemic hypertension, including EIH.
Mode of intervention
Considerations when deciding whether to proceed with open or endovascular repair include—patient age, physiologic status, frailty and comorbidities. We favor open repair in young, healthy individuals or those with prior endovascular repair with recurrent coarctation deemed to be at low risk for open surgery. We favor endovascular repair in poor open surgical candidates or those with previous open repair with recurrent coarctation. Aneurysm, pseudoaneurysm or other complicating factors require patient-specific consideration, such as proximity to head vessels, risk of rupture/dissection, etc. The presence of additional cardiac or aortic pathology, such as an incompetent aortic valve or ascending aortic aneurysm affects planning, and a hybrid approach may be chosen—for example, aortic valve and ascending aorta replacement through a sternotomy and endovascular coarctation repair.
For open repair, we primarily utilize Dacron interposition graft replacement of the coarcted segment via a left 4th interspace posterolateral thoracotomy. Unlike in the pediatric population, in the adult there is rarely enough mobility for adequate end-to-end repair, and, in our opinion, aortic continuity is superior to extra-anatomic bypass. We avoid patch repair, as it has been associated with increased risk of pseudoaneurysm formation and leaves the abnormal aortic segment in situ. Meticulous technique and dissection avoid injury to the esophagus, key nerves (vagus, recurrent laryngeal or phrenic), thoracic duct and large intercostal vessels and limits chest wall bleeding. Resection of residual ductal tissue, correct graft sizing and avoidance of graft kinking reduces the risk of turbulent, non-physiologic flow patterns. We emphasize aortic repair aesthetics or appearance (i.e., the fine art of aortic surgery, which is hard to quantify but leads to improved transition from native aorta to graft and improved flow dynamics).
Typically, we use left atrial to descending thoracic aortic bypass with a 16- or 18-Fr arterial cannula placed through a left inferior pulmonary vein purse string (Seldinger technique and transesophageal echocardiography confirmation). Outflow is generally a 16-Fr cannula in the descending aorta (Seldinger technique). While not always necessary due to chest wall collateralization, we feel that distal aortic perfusion (left atrium to descending thoracic aorta) is simple and facilitates meticulous repair. Rarely, full cardiopulmonary bypass for systemic cooling and circulatory arrest are necessary. Examples include hypoplastic arch associated with COA or aneurysmal dilation of a proximal landing zone or prior repair, where clamping is not possible. In very unusual circumstances, a more extensive exposure through a thoracosternotomy or clamshell are required to gain access to the ascending, arch and descending aorta simultaneously.
Options for endovascular repair are balloon angioplasty, bare metal stent and endograft placement, although we prefer the latter two options. A percutaneous approach is preferred, using whichever iliofemoral artery is more favorable. The preoperative CTA is pre-loaded into the hybrid endo-suite workstation, where it is fused as an overlay onto the patient, providing our road map for the procedure. Minor adjustments are made due to displacement of the anatomy by stiff guidewires. Ultrasound guidance is used for femoral artery access. Intravascular ultrasound is performed to define anatomy, adjust fusion image if needed and confirm the pre-operative CT measurements in the dynamic state. If an invasive pressure gradient is needed, it is performed at this time.
We prefer a 0.035 double curved Lunderquist wire (Cook Medical Inc., Bloomington, Indiana, USA) or Amplatz wire for stent or stent graft delivery. For primary stent angioplasty we use a 16-Fr by 65 cm dry seal sheath (W.L. Gore & Associates Inc., Newark, Delaware, USA). In complex and tortuous anatomies, left radial access with a wire into the ascending aorta helps to mark the left subclavian artery. Depending on the tortuosity and the length of the coarctation, we choose either 40−10 or 50−10 Palmaz bare metal stents (Cordis Corporation, Santa Clara, California, USA) [Cheatham Platinum stents (NuMed Inc., Hopkinton, New York, USA) can be used as well]. The Palmaz stent is mounted onto a Coda (Cook Medical Inc.), Tyshak (B. Braun, Melsungen, Germany), Z-med (Bard Inc., Murray Hill, New Jersey, USA) or BIB (Bard) balloon. The BIB balloon is preferred when there is angulation, tortuosity or significant proximal and distal diameter discrepancy across the coarctation. The Palmaz stent will foreshorten once expanded beyond a 10-mm diameter, and therefore care must be taken to appropriately extend the stent proximal to the stenosis. We employ rapid ventricular pacing in some patients to reduce systolic blood pressure to under 60 mmHg and thus reduce the risk of stent movement during deployment. For endograft placement, we prefer to deploy the thoracic endovascular aortic repair (TEVAR) graft first and then post dilate with the Palmaz stent. The endograft is deployed, then post-dilated with a compliant balloon, such as a Coda or a Reliant (Medtronic, Minneapolis, Minnesota, USA) with hand inflation. The treated area is re-evaluated by pressure gradient, followed by intravascular ultrasound and then a completion arteriogram. Occasionally, we will perform multiple planned balloon dilatations of the Palmaz stent at separate endosuite sessions when confronted with an especially tight stenosis. We feel dilation over time is safer than immediate full expansion in a native primary coarctation to reduce the risk of pseudoaneurysm or rupture.
Due to potential long-term complications previously discussed, we obtain follow-up imaging, either CTA or MRA, at one month, six months, one year and every five years thereafter regardless of the selected approach (15). Patients must continue to be followed for guideline directed management of their hypertension as well (2).
Results
During the study period, twenty-four adult patients with COA were treated at our center. The average age at intervention was 45.3±13.2 years, and 12 (50%) were male. Three (12.5%) patients had recurrent coarctation, re-stenosis or a complication of a prior coarctation procedure. Nine (38%) patients underwent open procedures, and 15 (62%) underwent endovascular procedures. Procedural details are summarized in Table 1. The procedural success rate was 100%. There were no incidences of postoperative stroke or renal failure. There were no mortalities up to five years following the procedure. One patient had a late death (after five years) from non-aortic causes, and five were lost to follow-up, but not prior to at least one year after the index intervention. At the time of most recent patient contact, 32% of patients (6 of 19) were normotensive and off antihypertensive medications.
Table 1
| Case | Approach | Device | Distal aortic perfusion | BP at last contact |
|---|---|---|---|---|
| 1 | Endo | Palmaz XL (40×10) | N/A | On medication |
| 2 | Open | Dacron (22 mm) | DHCA with ACP | On medication |
| 3 | Open | Dacron (20 mm) | None | Normotensive, off medication |
| 4 | Endo | Zenith TBE (32×32×80); did not reduce gradient; Palmaz T40-10XL in Zenith cuff; post-dilate Cordis Maxi 25 mm | N/A | Normotensive, off medication |
| 5 | Endo | Valiant (34×90) | N/A | On medication |
| 6 | Open | Dacron (22 mm) | LA—distal thoracic Ao | Unknown |
| 7 | Endo | Palmaz XL (40×10) ×2 | N/A | Unknown |
| First stent migrated | ||||
| 8 | Open | Dacron (18 mm) | LA—distal thoracic Ao | On medication |
| 9 | Endo | Palmaz XL (40×10) | N/A | Unknown |
| 10 | Endo | PTA of prior Palmaz | N/A | Unknown |
| 11 | Endo | Palmaz XL (40×10) | N/A | On medication |
| 12 | Open | Dacron (22 mm) | LA—distal thoracic Ao | On medication |
| 13 | Endo | Gore CTAG (21×10) | N/A | On medication |
| Post-dilate PTA (14×4) | ||||
| 14 | Open | Dacron (22 mm) | None | On medication |
| 15 | Endo | Gore CTAG (21×10) | N/A | On medication |
| Palmaz XL (40×10) within CTAG | ||||
| 16 | Endo | Palmaz XL (49×10) | N/A | On medication |
| 17 | Endo | Palmaz XL (49×10) | N/A | On medication |
| 18 | Open | Hemashield (14 mm) | None | Normotensive, off medication |
| 19 | Endo | Palmaz XL (50×10) | N/A | Normotensive, off medication |
| 20 | Endo | Palmaz (40×10) | N/A | Unknown |
| Atlas (16×4) | ||||
| 21 | Endo | Palmaz XL (49×10) | N/A | Normotensive, off medication |
| 22 | Open | Dacron patch aortic angioplasty | DHCA with ACP | On medication |
| 23 | Open | TEVAR extirpation | HCA, ACP, side-arm distal perfusion | On medication |
| Gelweave single branch (22 mm) | ||||
| Gelweave multibranch (26 mm) | ||||
| 24 | Endo | Amplatzer plug of coarctation; Gore CTAG (31×15) in old bypass; Gore TAG (37×15) within CTAG | N/A | Normotensive, off medication |
Ao, aorta; ACP, antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest; HCA, hypothermic circulatory arrest; LA, left atrium; PTA, percutaneous transluminal angioplasty.
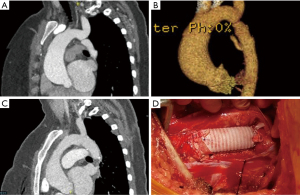
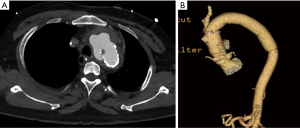
Several illustrative or atypical open surgical cases were identified. Figure 1 demonstrates a typical example of preoperative and postoperative imaging of COA at the aortic isthmus just distal to the left subclavian artery; an intraoperative photograph of the interposition graft is also included. In an atypical case, a 41-year-old man who underwent open patch angioplasty as a child later underwent TEVAR for a pseudoaneurysm at the site of repair (Figure 2). Subsequently, he developed a large, infected fluid collection around the aortic arch and proximal descending thoracic aorta necessitating reoperation with extirpation of all graft material and replacement of the ascending aorta, arch and descending aorta. The approach was through a thoracosternotomy.
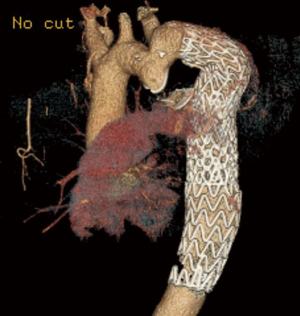
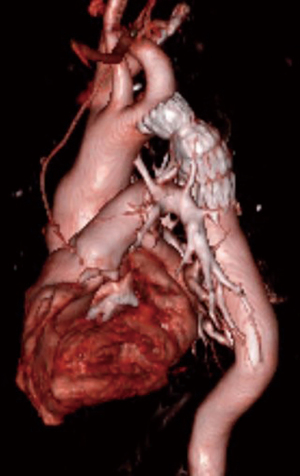
Several endovascular cases of interest were also identified. The first was a patient with COA previously treated by left subclavian artery to descending aorta bypass (Figure 3). The descending aorta distal to the graft became aneurysmal. An Amplatzer device was used to occlude the patent native coarct, and a Gore CTAG stent graft was placed in the aneurysmal aorta using the prior bypass graft as the proximal landing zone, as previously described in detail (16). This case is a cautionary tale as to why we favor interposition graft over extra-anatomic bypass—dealing with the patent residual native coarct can complicate redo endovascular approaches. Second, a more routine case of COA repaired with a covered stent that required additional Palmaz stent placement to achieve an acceptable post-repair gradient is depicted in Figure 4.
Discussion
Herein, we presented our single-center experience with management of COA in twenty-four adult patients over ten years, including our multidisciplinary approach to work-up and treatment. We have had no 30-day, one-year or five-year mortalities. Despite technical success in all cases, nearly 70% of patients remained on antihypertensives at their last contact. Our experience mirrors the limited data available in the literature.
Open repair has outstanding early postoperative morbidity and mortality (<1%) and early resolution of hypertension in 13–76% of patients. This approach has a positive impact on the natural history of the disease, extending the lifespan of treated patients (2,6). Follow-up imaging is important, as post-surgical repair problems include re-coarctation, aneurysm at the proximal or distal graft-to-aorta suture lines or pseudoaneurysm (4,15). More recently, endovascular therapy for COA has proven to be a safe and effective tool. Aortic wall rupture and aortic dissection are uncommon immediate procedural risks, which can be minimized with proper preoperative planning and stent selection. Re-stenosis, re-coarctation and aneurysm formation are important long-term complications that emphasize the need for long-term imaging follow-up after endovascular repair as well.
Our center has achieved a post-intervention peak-to-peak gradient less than 20 mmHg in all patients undergoing open surgical or endovascular repair of COA. However, the marked variable resolution of systemic arterial hypertension in our patient cohort mirrors reports from other centers (up to 18–88%) (5). Very long-term follow-up has demonstrated that 40–55% of patients are hypertensive at twenty years after surgical repair of COA (1,2). Changes in aortic wall compliance and flow resistance within the surgical graft following open repairs remain a potential area of improvement. Perhaps with improved biomimetic materials, these limitations can be ameliorated in the future. As with surgery, persistent or recurrent hypertension after endovascular repair is common and requires long-term attention. Often to our chagrin, we see no change in the number of antihypertension medications taken pre- or post-intervention. Perhaps earlier intervention to prevent irreversible vascular remodeling could improve these results. These findings should provide further impetus for early intervention and multidisciplinary long-term follow-up to optimize care of this uncommon clinical problem in the adult.
The obvious limitation of our paper is the single center experience which prevents us from making any broad generalizations or definitive recommendations. We have not compared outcomes between the cohorts of patients treated with open surgical versus endovascular repair because of clear treatment selection bias and the low number of post-intervention events. However, our results, at least in terms of technical success, short- and mid-term mortality and avoidance of complications, are good and should give confidence when counseling patients with COA. We attribute this to the heart team approach and protocols that we have outlined above.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hager A, Kanz S, Kaemmerer H, et al. Coarctation Long-term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg 2007;134:738-45. [Crossref] [PubMed]
- Musto C, Cifarelli A, Pucci E, et al. Endovascular treatment of aortic coarctation: long-term effects on hypertension. Int J Cardiol 2008;130:420-5. [Crossref] [PubMed]
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv 2010;76:553-63. [Crossref] [PubMed]
- Cardoso G, Abecasis M, Anjos R, et al. Aortic coarctation repair in the adult. J Card Surg 2014;29:512-8. [Crossref] [PubMed]
- Hamid T, Motwani M, Schneider H, et al. Benefit of endovascular stenting for aortic coarctation on systemic hypertension in adults. Arch Cardiovasc Dis 2015;108:626-33. [Crossref] [PubMed]
- Roselli EE, Qureshi A, Idrees J, et al. Open, hybrid, and endovascular treatment for aortic coarctation and postrepair aneurysm in adolescents and adults. Ann Thorac Surg 2012;94:751-6; discussion 757-8. [Crossref] [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:1494-563. [Crossref] [PubMed]
- Crafoord C, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Surg 1945;14:347-61. [Crossref]
- Campbell M. Natural history of coarctation of the aorta. Br Heart J 1970;32:633-40. [Crossref] [PubMed]
- O'Laughlin MP, Perry SB, Lock JE, et al. Use of endovascular stents in congenital heart disease. Circulation 1991;83:1923-39. [Crossref] [PubMed]
- Erben Y, Oderich GS, Duncan AA. Endovascular repair of aortic coarctation pseudoaneurysm using an off-label "hourglass" stent-graft configuration. J Endovasc Ther 2015;22:460-5. [Crossref] [PubMed]
- Karaosmanoglu AD, Khawaja RD, Onur MR, et al. CT and MRI of aortic coarctation: pre- and postsurgical findings. AJR Am J Roentgenol 2015;204:W224-33. [Crossref] [PubMed]
- Katz G, Uretzky G, Beer G, et al. Long-term results of surgical repair of coarctation of the aorta--evaluation by exercise test. Cardiology 1987;74:465-73. [Crossref] [PubMed]
- Egbe AC, Warnes CA, Connolly HM. Critical Appraisal of the Indications for Intervention in Adults With Coarctation of Aorta. J Am Coll Cardiol 2020;75:1089-90. [Crossref] [PubMed]
- Krupiński M, Irzyk M, Moczulski Z, et al. Morphometric evaluation of aortic coarctation and collateral circulation using computed tomography in the adult population. Acta Radiol 2020;61:605-12. [Crossref] [PubMed]
- Baumgarten H, Squiers JJ, Brinkman WT, et al. Endovascular Technique for Repair of Descending Thoracic Aortic Aneurysm After Coarctation Operation. Ann Thorac Surg 2017;103:e167-9. [Crossref] [PubMed]






