Total arch replacement using selective antegrade cerebral perfusion as the neuroprotection strategy
Introduction
Results of surgery have steadily improved over the past few decades due to advancement of surgical technologies, deeper knowledge of the disease, utilization of sealed grafts, improved protection of the central nervous system, development of stent-grafting, and evolution of surgical skills and techniques (1-4). According to a survey by the Japanese Association for Thoracic Surgery, since 1984, the annual number of operations for the thoracic aorta has dramatically increased from 712 in 1984 to 12,439 in 2010 (5). Thirty-day mortality after elective arch replacement has decreased to 7.0%, while hospital mortality after emergent operation for acute type A aortic dissection was reported to be 9.2% in a 2010 Japanese registry. At the same time, new strategies for treating aortic disease have emerged, including endovascular or hybrid stent-grafting. It has become imperative to devise not only surgical techniques, but also accurate preoperative imaging for aortic pathology, monitoring of adequate brain perfusion, maintenance of strict fluid balance during cardiopulmonary bypass (CPB), and pharmacological interventions for pulmonary protection. Such developments may lead to further improvement in short- and long-term outcomes for the treatment of aortic arch disease.
Patients and methods
From January 2002 to December 2012, 1005 patients underwent aortic arch replacement at Kobe University Hospital, which consisted of 404 patients with ascending aorta to hemiarch replacement, 497 with total arch replacement, 72 with distal arch replacement, and 32 with extensive arch replacement. In this study, 438 patients who underwent total arch replacement using selective antegrade cerebral perfusion were investigated. The mean age of the patients was 69.1±13.4 years and 83 (18.9%) were over 80 years of age. 73.7% of patients were male. Aortic pathology was non-dissection aneurysm in 280 (63.9%) and aortic dissection in 159 (36.3%), including acute type A dissection in 80 (18.3%) and type B complicated with non-dissection aneurysm in 6 (1.3%). Thirty patients (6.8%) had aortitis syndrome, infected aneurysm, and other pathologies. Shaggy aortic intima in the aortic arch was observed in 36 patients (8.2%). There were 16 Marfan patients (3.7%). Surgery was performed on emergent/urgent basis in 144 patients (32.9%), including 36 (8.2%) ruptured cases. Fourteen patients (3.2%) were in shock and underwent cardiopulmonary resuscitation. Other conditions included a history of cerebral infarction in 35 patients (8.0%), 94 patients (21.5%) with coronary artery disease, 35 (8.0%) with chronic obstructive pulmonary disease, 15 (3.4%) with left ventricular dysfunction (ejection fraction <40%) and 35 (8.0%) with chronic kidney disease (serum creatinine ≥2.0 mg/dL). The average EuroSCORE was 8.8±3.1 (Table 1).
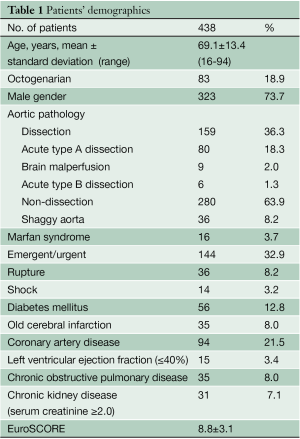
Full table
Surgical approach
Our current approach has been (I) meticulous selection of arterial cannulation site and type of arterial cannula; (II) selective antegrade cerebral perfusion (SACP) for cerebral protection; (III) whole body hypothermia with minimal tympanic temperatures between 20 and 23 °C and minimal rectal temperatures below 30 °C; (IV) early re-warming after distal anastomosis with SACP flow adjustment while monitoring brain oxygenation with near infrared spectroscopy (NIRS); and (V) after 2006, maintaining strict fluid balance below 1,000 mL by the extracorporeal ultrafiltration method (ECUM) during CPB, with the expectation of more rapid pulmonary functional recovery (6).
Epi-aortic echo scanning was routinely performed. Selection of cannulation site and type of arterial cannulation for CPB was particularly important. Preoperative CT scan was done in every patient, including emergency cases, to assess the atheromatous lesions in the ascending aorta. Both transesophageal and epi-aortic echography were applied to interrogate the ascending aorta and determine cannulation site. Selected cannulation sites included the ascending aorta/arch (331; 75.1%), the femoral artery (71; 16.2%), the axillary artery (1; 0.2%), the femoral artery + axillary artery (8; 1.8%), and the ascending aorta + femoral artery (25; 5.7%) (Table 2).
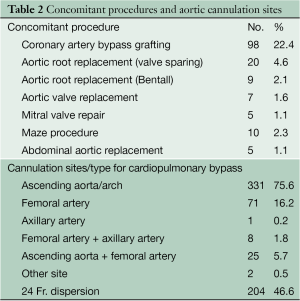
Full table
CPB was established with bicaval drainage. The left ventricle was vented through the right upper pulmonary vein. Femoral artery cannulation was applied particularly in patients with aortic dissection. For diseased ascending aorta/aortic arches, a 24 Fr dispersion arterial cannula (Duraflo II, Edwards Lifesciences LLC, Irvine, CA, USA) was used in 204 cases (46.6%). Other patients had a straight tip cannula in the ascending aorta (DLP, Medtronic, Minneapolis, MN, USA). The tip of catheter was always set to the aortic valve in order to avoid direct flow to the arch. All patients received 100 mg of betamethasone sodium phosphate, and 100 mg of sivelestat sodium hydrate added to the pump circuit at the initiation of CPB. Further details of operative strategies are described in the Art of Operative Techniques articles in this issue. Other concurrent procedures are shown in Table 2.
Definition of neurological deficits
Permanent neurological dysfunction (PND) was defined as the presence of deficits that persisted at discharge which was caused by intraoperative procedure. Transient neurological dysfunction (TND) was defined as transient loss of orientation, slurred language, agitation, or poor response to commands. Neurological dysfunction caused by preoperative brain malperfusion associated with acute type A dissection, deep shock status or postoperative atrial fibrillation were excluded from this category (7). Brain malperfusion was defined as cerebral blood flow disturbance secondary to acute aortic dissection involving aortic arch branches with newly developed stroke, transient ischemic attack, or consciousness disorder.
Statistical analysis
Data was processed using Stat View J-5.0 software (SAS Institute, Cary, NC, USA). Continuous values were expressed as the mean ± standard deviation. Categorical variables were analyzed by the chi-squared test. Stepwise logistic regression analysis was performed to identify the risk factors for hospital mortality, PND and TND. Clinically relevant variables with P<0.05 on univariate analysis were incorporated into the multivariate models. Survival and freedom from aortic related death was assessed by the Kaplan-Meier method. Differences were considered statistically significant at P<0.05.
Results
Operative data
Mean CPB time, myocardial ischemic time, lower body circulatory arrest time, SACP time, and minimum tympanic and rectal temperatures were 191±69 minutes, 92±50 minutes, 44±15 minutes, 100±33 minutes, 23.5±2.7 °C and 26.9±3.0 °C, respectively. In 39 patients who had arch-first reconstruction, mean CPB time, myocardial ischemic time, and SACP time were 183±73, 124±56, and 79±34 minutes, respectively. Mean lower body circulatory arrest time in patients with acute aortic dissection was 51±16 minutes, which was significantly lower because there was a necessity to insert and fix the elephant trunk in the descending aorta (Table 3).
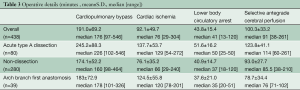
Full table
Perioperative data
Thirty-day mortality was 3.0% (13/438), and was 7.7% (11/144) in urgent/emergent surgery, and 0.68% (2/294) in elective cases (Table 4). In-hospital mortality was 4.6% overall (20/438), and was 9.7% (14/144) in urgent/emergent surgery, and 2.0% (6/294) in elective cases. The causes of death included: central nervous system (n=7), pulmonary problems (n=6), sepsis (n=2), necrosis of the intestine (n=2), hemorrhage (n=2), and acute myocardial infarction (n=1). Multivariate analysis demonstrated that risk factors for hospital mortality were octogenarian (OR 4.45, P=0.03), brain malperfusion due to acute aortic dissection (OR 23.52, P=0.002) and prolonged cardiopulmonary bypass time (OR 1.07, P=0.04, Table 2).
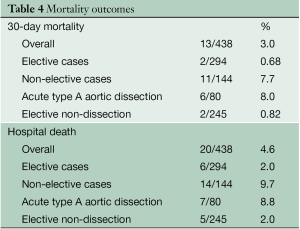
Full table
PND occurred in 23 patients (5.3%) following surgery, and in 8 patients (2.7%) operated on electively. An additional 5 patients had a neurological deficit preoperatively, caused by brain malperfusion in 4 and by deep shock status in one patient, which from left main trunk malperfusion associated with acute type A aortic dissection. Three patients had stroke secondary to postoperative atrial fibrillation. TND occurred in 38 patients (8.7%) following surgery, and in 11 patients (3.7%) operated on electively. Additional postoperative complications are shown in Table 5. Mean mechanical ventilation time was 23.2±39.7 hours. 58 patients (13.2%) required prolonged mechanical ventilation (≥48 hours).
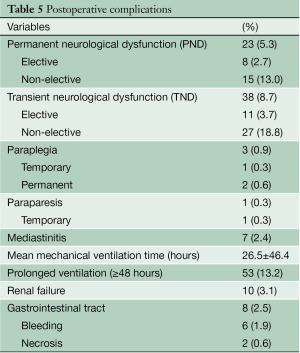
Full table
Survival data
Follow-up was completed in 97.2% of patients, with mean follow-up period of 2.3±2.3 years. Late death occurred in 51 patients; causes of death included pneumonia (n=19), malignancy (n=8), cardiac event (n=7), cerebrovascular event (n=5), aorta-related event (n=4), gastrointestinal problem (n=3), renal failure (n=2), and others (n=3). The four aorta-related events included rupture of the penetrating atherosclerotic ulcer in the descending aorta 1 year after arch replacement, rupture of thoracoabdominal aortic aneurysm 3 years after surgery, sudden death with hemoptysis presumably caused by aorto-pulmonary fistula, and sudden death with unknown reason 4 months after surgery.
Overall long-term survival was assessed by the Kaplan-Meier method. Survival at 5 and 10 years after surgery was 79.6±3.3% and 71.2±5.0%, respectively. In the acute type A dissection group, survival at 10 years was 96.8±2.9%. In the elective non-dissection group, survival was 81.4±7.2% at 5 years and 77.0±5.9% at 10 years.
Reoperation data
Twenty-three patients required repeat (n=17) or additional (n=6) aortic surgery. Repeat surgery was necessary in two patients who had proximal false aneurysm secondary to GRF glue necrosis, in two who had distal false aneurysm, and in two with due to elephant trunk infection or kinking. Freedom from repeat aortic surgery was 98.4±2.5% at 5 years and 91.7±7.1% at 10 years. Additional surgery consisted of aortic root/valve reconstruction (n=3), replacement of the descending aorta (n=3), thoracoabdominal aorta (n=7), and thoracic endovascular aortic repair (n=5). Freedom from additional aortic surgery was 95.4±3.5% and 88.5±7.7% at 5 years and 10 years, respectively.
Discussion
Recent advancements in surgery for the aortic arch is widely recognized. This achievement has been attributed to the development of water-sealed grafts, evolution of CPB techniques and equipment, better understanding of brain protection, and refinement of the surgical procedure details (8,9). We have published our initial experience of aortic arch surgery previously (7). We found that risk factors for early mortality in patients who underwent total arch replacement were octogenarian, preoperative brain malperfusion, and prolonged CPB. In elective patients without dissection, risk factors for postoperative stroke were leukoariosis and shaggy aorta. Risk factors for TND include shaggy aorta, leukoaraiosis, extracranial stenosis of the carotid arteries, and prolonged CPB time (10).
The severity of aortic arch atheroma grades has been identified as another risk factor for adverse neurological outcomes (10). Amarenco et al. (11) indicated a strong, independent association between atherosclerotic disease of the aortic arch and the risk of ischemic stroke, particularly with thick plaque (≥4 mm). Multivariate analysis demonstrated that shaggy aorta was a risk factor for TND, but not for PND. We believe that meticulous selection of cannulation site and the type of cannula used, based on the pre- and intra-operative accurate imaging for aortic pathology and complete exclusion of the diseased aorta, contributed to avoiding PND even in cases with shaggy aorta.
The temperature of hypothermic circulatory arrest during the distal anastomosis is particularly crucial for protecting vital organs. Since the primary goal of core cooling is to achieve a brain temperature compatible with the maximum suppression of metabolism, the lowest temperatures that can be safely achieved is optimal for brain protection (12). On the other hand, deep hypothermia tends to be associated with coagulopathy or lung injury, which is to be avoided. The optimal temperature in the setting of SACP has been reported to be between 20 and 28 °C (2,4,12). Kamiya et al. (13) reported that the temperature during hypothermic circulatory arrest could be safely increased to 28 °C with a high SACP flow rate, and that the incidence of neurological events was not increased. We set tympanic temperature between 20 and 23 °C, resulting in optimal circulatory arrest and SACP time (42.4±27.8 and 97.3±31.4 minutes respectively). Rectal temperature was targeted below 30 °C just prior to circulatory arrest, as one of our patients, whose rectal temperature before circulatory arrest was in excess of 30 °C, had entire colon necrosis, presumably because of the presence of calcified stenosis of the mesenteric artery causing poor visceral circulation and protection.
In order to minimize CPB time, we started re-warming as soon as the distal anastomosis was completed, with concurrent antegrade distal reperfusion to the lower body through the prosthetic graft branch. We then moved to the proximal anastomosis. Usually immediately after arch vessel anastomosis, CPB was terminated. Monitoring brain oxygenation during re-warming is particularly important. We increased SACP flow from 10 to 15 mL/min/m2 to maintain preoperative values of NIRS (14). However, the total flow was always maintained below 1,200 mL/min to avoid brain edema.
The present study reported the surgical outcomes of total aortic arch replacement using SACP. Our approach for total arch replacement has contributed to low hospital mortality and morbidities leading to favorable long-term outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31.
- Strauch JT, Spielvogel D, Lauten A, et al. Technical advances in total aortic arch replacement. Ann Thorac Surg 2004;77:581-89; discussion 589-90.
- Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553-8.
- Kazui T, Washiyama N, Muhammad BA, et al. Improved results of atherosclerotic arch aneurysm operations with a refined technique. J Thorac Cardiovasc Surg 2001;121:491-9.
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010 : annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012;60:680-708.
- Okada K, Omura A, Kano H, et al. Recent advancements of total aortic arch replacement. J Thorac Cardiovasc Surg 2012;144:139-45.
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90; discussion 1891-4.
- Ogino H, Sasaki H, Minatoya K, et al. Evolving arch surgery using integrated antegrade selective cerebral perfusion: impact of axillary artery perfusion. J Thorac Cardiovasc Surg 2008;136:641-8; discussion 948-9.
- Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg 2007;83:S796-8; discussion S824-31.
- Morimoto N, Okada K, Uotani K, et al. Leukoaraiosis and hippocampal atrophy predict neurologic outcome in patients who undergo total aortic arch replacement. Ann Thorac Surg 2009;88:476-81.
- Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med 1994;331:1474-9.
- Asano M, Okada K, Nakagiri K, et al. Total arch replacement for aneurysm of the aortic arch: factors influencing the distal anastomosis. Interact Cardiovasc Thorac Surg 2007;6:283-7.
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9.
- Olsson C, Thelin S. Regional cerebral saturation monitoring with near-infrared spectroscopy during selective antegrade cerebral perfusion: diagnostic performance and relationship to postoperative stroke. J Thorac Cardiovasc Surg 2006;131:371-9.





