Pulmonary thromboendarterectomy in chronic thromboembolic pulmonary hypertension: the Spanish experience
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is an infrequent evolution of acute pulmonary embolism (PE). Its prevalence varies from 0.57% to 9.1% (1); and several mechanisms behind the development of CTEPH have been suggested (2). It is a rare disease, associated with high morbidity and mortality when not treated (3,4). It is still underdiagnosed but slowly starting to gain visibility. It should be diagnosed in its early phases so that CTEPH patients can be offered the best possible treatment (1,5,6). Nowadays it is a potentially curable disease (6,7), with pulmonary endarterectomy (PEA) being considered the treatment of choice in a high number of patients. This intervention pursues three main goals: (I) hemodynamic stability, reducing the effect of pulmonary hypertension (PH) on the right ventricle by preventing right ventricular failure and secondary valvular disease; (II) respiratory stability, by improving ventilatory efficiency; and (III) improved exercise capacity (1,5,8). PEA is a technically demanding operation, currently only performed in very few selected centers around the world; optimal results are associated with better patient selection, better perioperative care and greater surgical experience (1,5,8). When performed in specialized centers, better results are found in terms of patient survival, functional class and exercise capacity due to the improvement of hemodynamics after the surgery (4,7,9-11). Furthermore, the ability of PEA to allow access to the lesions does not only depend on their anatomical distribution, but also on the surgeon’s previous experience (2). There is a group of patients considered inoperable (around 35–40% of CTEPH patients) (6,12), who have two other treatments options: balloon pulmonary angioplasty (BPA), and PH-targeted medical therapy (MT) with riociguat (a stimulator of soluble guanylate-cyclase enzyme) (13), which was approved in 2015 in Spain for patients with inoperable CTEPH or persistent PH after PEA.
National Spanish outcomes in CTEPH management are scarce due to the current decentralized model (1,6). Currently, CTEPH can be treated in PH specialized centers or at general hospitals, where the decision of referring the patient for surgery is left to the treating doctor (1). However, all patients should be referred and carefully evaluated by a Multidisciplinary Expert Team, where every individual case is discussed, and the most suitable treatment is chosen. The Multidisciplinary Expert Team is made up of pulmonologists, cardiologists, radiologists and cardiac surgeons specialized in PEA (9). There are two PH specialized centers in Spain that bring together most CTEPH patients (>60%), designated as Centros, Servicios y Unidades de Referencia del Sistema Nacional de Salud (CSUR): Hospital Clínic, Barcelona, and Hospital Universitario 12 de Octubre, Madrid (5,14). These centers assess patients who belong to the corresponding health administrative area and those being referred from general hospitals (1). A recently published study shows that a low percentage of patients were referred to CSUR in Spain (61.4%), which led to a lower rate of total PEA (30.7%), and higher overall mortality (1). Furthermore, it is important to mention that, since 2007, a national observational registry of pulmonary hypertension (REHAP) has been running in order to evaluate national clinical management of CTEPH patients and its long-term outcomes in Spain (1,6). Both specialized centers participate in the REHAP Registry and in the International CTEPH Registry (14).
Our objective is two-fold: (I) to investigate demographics, echocardiographic and hemodynamic characteristics of the 338 CTEPH patients who underwent surgery in CSUR Centers in Spain; and (II) to analyze surgical outcomes, immediately and up to one-year after the surgery. In-hospital and long-term mortality were analyzed. With this data, we want to reinforce our idea of changing the current model of CTEPH management in Spain.
Methods
Inclusion criteria
In the aforementioned centers, 578 patients between January 1st 2007 and December 31st 2019 met the definition of CTEPH, fulfilling therefore, the specific hemodynamic criteria for right heart catheterization (RHC): mean pulmonary artery pressure (mPAP) ≥25 mmHg, pulmonary vascular resistance (PVR) ≥3 Wood units or ≥240 dyn.s.cm−5 and pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, or below this level but with documented exercise PH. Of the evaluated patients, 338 were considered operable, the remaining were deemed inoperable and given other treatment options. Moreover, all patients (operable and inoperable) showed perfusion defects in ventilation/perfusion lung scintigraphy and CT angiography, consistent with CTEPH. They all received at least three months of anticoagulation treatment before the final diagnosis of CTEPH was given and continued receiving it long term. Data such as demographic and anthropometric parameters, PH clinical characteristics and supplementary diagnostic tests parameters (echocardiographic and RHC variables) (Table 1) were obtained from routine medical visits.
Table 1
| Variables | Mean ± SD or n (%) |
|---|---|
| Demographic and anthropometric | |
| Age (years) | 53.5±15.0 |
| Male gender | 182 (53.8) |
| BMI (kg/m2) | 28.0±5.2 |
| Systemic hypertension | 91 (35.0) |
| DM | 25 (9.6) |
| Current/past smoking habit | 105 (40.4) |
| Coronary artery disease | 16 (6.2) |
| Cancer history | 25 (9.7) |
| Hypercoagulability | 115 (34.0) |
| PE history | 290 (85.8) |
| DVT | 153 (45.3) |
| Clinical | |
| WHO I–II | 101 (31.5) |
| WHO III–IV | 220 (68.5) |
| 6MWD (m) | 399.4±123.4 |
| NT-proBNP (mg/dL) | 1,403.3±2,034.9 |
| Hemodynamic and echocardiographic | |
| mPAP (mmHg) | 46.5±13.1 |
| PVR (dyn·s·cm−5) | 764.5±392.8 |
| RAP (mmHg) | 9.1±5.4 |
| PCWP (mmHg) | 10.3±4.0 |
| Cardiac index (L/min/m2) | 2.30±0.59 |
| TAPSE (mm) | 17.4±4.3 |
| Pericardial effusion | 32 (12.8) |
BMI, body mass index; DM, diabetes mellitus; PE, pulmonary embolism; DVT, deep vein thrombosis; WHO, World Health Organization; 6MWD, six-minute walk distance; NT-proBNP, N-terminal pro B-type natriuretic peptide; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
PEA selection
Most patients will benefit from PEA surgery, but the selection criteria remain subjective to each multidisciplinary team. It is based on several factors, such as lesion accessibility (anatomic distribution—assessed by CT angiography—and the cardiac surgeon’s expertise), severity of the patient’s disease including symptoms and hemodynamic status (severity of PH and right heart dysfunction) and presence of co-morbidities, including long-term expectations (1,12). Therefore, the selection of candidates for surgery depends on the combination of accessible surgical disease and the severity of PH and right ventricular dysfunction, but neither the severity of right ventricular dysfunction nor the value of PVR will exclude a patient from surgical consideration, however, the PEA and postoperative care are made more challenging. In terms of accessibility, if the disease is in the main, lobar or segmental pulmonary artery branches, endarterectomy is feasible; and it is only performed in the most expert centers even if subsegmental arteries are primarily affected (1,2,5,6,12). Nevertheless, distal segmental disease is much more difficult to remove and renders the patient inoperable (7,12,15). The decision to operate was always made in a meeting of members of the Multidisciplinary Pulmonary Hypertension Team.
Surgical technique and follow -up
PEA was performed in accordance with the University of California’s protocol (San Diego, USA) (12). Surgical approach was through median sternotomy, and performed with full cardiopulmonary bypass, aortic cross-clamping and deep, hypothermia. Endarterectomy was performed during ten-minute periods of circulatory arrest, followed by five-minute reperfusion lapses. The extracted material during the PEA was grouped according to histopathological prognostic value established by the University of California group (16): type 1, fresh thrombus in the main-lobar pulmonary arteries; type 2, intimal thickening and fibrosis proximal to the segmental arteries, with no thrombus; type 3, disease within distal segmental arteries only; and type 4, distal arteriolar vascular disease. In-hospital mortality and all causes of death were collected. Postoperative complications were described as presence of reperfusion edema (postoperative respiratory failure causing hypoxia, accompanied by pulmonary infiltrates on chest X-ray in some of the surgical areas, occurring in the first 72 hours after PEA, and needing mechanical ventilation for more than 96 hours), cardiac failure, need for extracorporeal membrane oxygenation (ECMO), neurological complications and residual PH. Both the definition of residual PH and the time for diagnosis are controversial; some authors establish it according to mPAP values and others to PVR values. In our study, residual PH was defined as having mPAP >25 mmHg at rest, and clinically relevant PH as having PVR >400 dyn·s·cm−5 (in the six-month RHC). All patients remained on long-term anticoagulant therapy. Follow-up time was at least one year.
Statistical analysis
Continuous variables were expressed as a mean ± standard deviation, or as a median with interquartile range (IQR) when not normally distributed. Quantitative variables were analyzed using the Student’s t-test or Mann-Whitney U-test. Comparisons were made using the paired t-test or ANOVA. Categorical variables were expressed as frequencies, n (%) and compared using the Chi-square, Fisher’s exact test or McNemar’s for paired samples. All P values were two-sided, with a P value <0.05 being considered statistically significant. Preoperative, intraoperative, and postoperative variables were analyzed to evaluate if they statistically correlated with in-hospital mortality using logistic regression; those that were available in less than a 70% of patients were excluded [such as N-terminal pro B-type natriuretic peptide (NT-proBNP) which was firstly measured in 2009 in one of the two expert centers]. Multivariable logistic regression analysis was performed for variables considered risk factors, to calculate their relative risk and 95% confidence interval (CI). The univariate Cox regression model was used to evaluate proportional hazards for mortality, and those that revealed a P<0.05 significance were included in the multivariable analysis (again NT-proBNP was not included in this Cox analysis). The proportional hazards assumptions were checked using scaled Shoenfeld residual, using both hypothesis testing and graphical methods. The linearity assumptions were checked by plotting the Martingale residuals against continuous covariates. Survival curves were calculated using the Kaplan-Meier method and compared using the log rank test. The study date of entry was defined as the date of the first diagnostic RHC. The statistical analysis was performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA) and R software version 4.1.1.
Results
Study population and situation at diagnosis
The study population included a group of 338 patients with CTEPH who met the inclusion criteria and underwent surgery between January 1st 2007 and December 31st 2019; constituting 58.5% of the total assessed patients across the two specialized centers. Clinical and hemodynamic characteristics at diagnosis of all patients are listed in Table 1; mean age was 53.5±15.0 years, 53.8% were men and most of patients had severe clinical disease [68.5% were in World Health Organisation (WHO) functional class III–IV] and a severe hemodynamic condition (with a mPAP of 46.5±13.1 mmHg and 80 patients (23.7%) had previous PVR >1,000 dyn·s·cm−5).
Immediate and one-year outcomes
PEA immediate postoperative outcomes
In patients who underwent PEA, mean CBP time was 215.8±47.0 minutes, mean cross-clamp time was 113.1±24.6 minutes, with a mean time of circulatory arrest of 42.8±14.5 minutes. Complete PEA was performed in 95.5% and concomitant surgery in 70 (20.6%) patients (Table 2). According to the San Diego classification system of the biological material extracted, 27.9% was classified as type 1, 49.8% as type 2 and 22.3% as type 3. Complications in postoperative care were registered; the global in-hospital mortality was 3.3%, postoperative reperfusion edema occurred in 14.2% of patients, cardiac failure in 6.5% and ECMO was required in 25 patients (7.4%), 18 of which were veno-venous and 7 were veno-arterial. Additionally, postoperative neurological complications occurred in 5.9% of patients, most of which were temporary (5.3%) but in two cases (0.6%) caused permanent damage. Median intensive care unit (ICU) stay was 6 (IQR 8) days; and in-hospital stay was 14 (IQR 12) days (Table 2). Patients’ preoperative, intraoperative and postoperative characteristics potentially associated with greater in-hospital mortality were assessed by logistic regression univariate analysis; the items found to be risk factors for in-hospital mortality are specified in Table 3. Following multivariable analysis, only previous PVR >1,000 dyn·s·cm−5 remained an independent risk factor for in-hospital mortality (P=0.010) (Table 3).
Table 2
| Variables | Mean ± SD or n (%) or median [IQR] |
|---|---|
| CPB time (min) | 215.8±47.0 |
| Cross-clamp time (min) | 113.1±24.6 |
| Circulatory arrest (min) | 42.8±14.5 |
| Complete PEA | 323 (95.5) |
| Concomitant surgery | 70 (20.6) |
| San Diego anatomo-surgical classification | |
| Type 1 | 89 (27.9) |
| Type 2 | 159 (49.8) |
| Type 3 | 71 (22.3) |
| ICU stay (days) | 6 [4–12.8] |
| In-hospital stay (days) | 14 [9–21] |
| In-hospital mortality | 11 (3.3) |
| Reperfusion edema | 48 (14.2) |
| Cardiac failure | 22 (6.5) |
| ECMO | 25 (7.4) |
| Neurological complications | 20 (5.9) |
CBP, cardiopulmonary bypass; PEA, pulmonary endarterectomy: ICU, Intensive Care Unit; IQR, interquartile range; ECMO, extracorporeal membrane oxygenator; SD, standard deviation.
Table 3
| Risk factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| RR [95% CI] | P | RR [95% CI] | P | ||
| WHO functional class IV | 10 [23–47] | 0.001 | |||
| RAP | 1.1 [1.0–1.2] | 0.034 | |||
| Cardiac output | 0.5 [0.2–0.9] | 0.042 | |||
| PVR >1,000 dyn·s·cm−5 | 8 [2–27] | 0.009 | 10.5 [1.8–85.0] | 0.010 | |
| Reperfusion edema | 11 [4–99] | <0.001 | |||
| Cardiac failure | 12 [2–57] | 0.001 | |||
| ECMO | 22 [5–150] | <0.001 | |||
WHO, World Health Organization; RAP, right atrial pressure; PVR, pulmonary vascular resistance; ECMO, extracorporeal membrane oxygenator; RR, relative risk; CI, confidence interval.
PEA one-year clinical and hemodynamic outcomes
One-year outcomes in PEA patients were assessed: both exercise capacity [6-minute walk distance (6MWD)] and clinical functional class (WHO classification) improved, and NT-proBNP values decreased significantly. Moreover, hemodynamic and echocardiographic features improved significantly after surgery: mPAP, RAP and PVR values decreased, and statistical differences were found between preoperative and postoperative figures. Additionally, both cardiac index and tricuspid annular plane systolic excursion (TAPSE) values improved after PEA (Table 4).
Table 4
| Variables | Preoperative | One-year outcomes | P (bilateral) |
|---|---|---|---|
| Clinical | |||
| WHO classification, n (%) | <0.001 | ||
| I–II | 76 (27.8) | 261 (95.6) | |
| III–IV | 197 (72.2) | 12 (4.4) | |
| 6MWD (m), mean ± SD | 403.9±113.0 | 466.5±96.3 | <0.001 |
| NT-proBNP (mg/dL), mean ± SD | 1315.3±1,589.5 | 288.6±322.7 | <0.001 |
| Hemodynamic and echocardiographic, mean ± SD | |||
| mPAP (mmHg) | 46.4±13.0 | 27.3±10.3 | <0.001 |
| PVR (dyn·s·cm−5) | 757.8±375.2 | 329.0±477.5 | <0.001 |
| RAP (mmHg) | 9.2±5.3 | 6.3±3.7 | <0.001 |
| PCWP (mmHg) | 10.6±4.0 | 10.5±6.7 | 0.932 |
| Cardiac index | 2.30±0.59 | 2.70±0.57 | <0.001 |
| TAPSE (mm) | 17.8±4.3 | 16.7±3.5 | 0.034 |
PEA, pulmonary endarterectomy; WHO, World Health Organization; 6MWD, six-minute walking distance; NT-proBNP, N-terminal pro B-type natriuretic peptide; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Residual PH after PEA
For patients whose data was available (n=260), 130 (50%) had persistent residual PH (mPAP >25 mmHg); and clinically relevant residual PH (PVR >400 dyn·s·cm−5) was seen in 59 (22.7%) patients. Mean preoperative PVR in the latter group of patients was 844.2±314.9 dyn·s·cm−5, higher than the rest of the patients.
PEA survival
During a mean follow-up of 38.5±27.3 months, there were a total of 27 deaths. One-, three- and five-year survival rates from diagnosis were 94.8%, 93.3% and 90.5%, respectively (Figure 1). Residual PH mortality was also evaluated; one-, three-, and five-year survival rates were 94.8%, 87.8% and 87.8%, respectively, and were slightly lower in those patients with normalized PVR after surgery, although, survival rates equalized six years after surgery (log-rank 0.004) (Figure 2). Univariate Cox regression was performed to evaluate mortality risk factors during follow-up (Table 5). For multivariable Cox analysis, only a higher 6MWD (P=0.009), therefore better physical capacity and higher cardiac output (by every 0.5 L/min increase) (P=0.033), remained an independent protective factor for mortality during follow-up (Table 5).
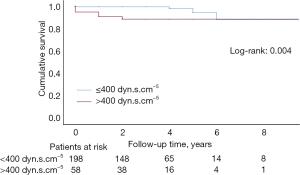
Table 5
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Demographic | |||||
| Age | 1.01 (0.99–1.07) | 0.156 | |||
| Female gender | 2.22 (1.20–11.02) | 0.01 | |||
| BMI | 0.95 (0.83–1.08) | 0.414 | |||
| DM | 0.04 (0.00–2.91) | 0.488 | |||
| Cancer history | 3.60 (1.57–10.58) | 0.009 | |||
| Current/past smoking habit | 1.26 (0.45–3.51) | 0.657 | |||
| Hypercoagulability | 1.01 (0.36–2.82) | 0.984 | |||
| PE history | 0.15 (0.06–0.55) | 0.002 | |||
| Clinical | |||||
| WHO functional class III-IV | 2.00 (0.45–9.00) | 0.214 | |||
| 6MWD (increase by 30 m) | 0.70 (0.65–0.85) | <0.001 | 0.80 (0.75–0.94) | 0.009 | |
| Hemodynamic | |||||
| RAP (increase by 10 mmHg) | 0.99 (0.40–2.35) | 0.973 | |||
| mPAP (increase by 10 mmHg) | 1.15 (0.77–1.76) | 0.475 | |||
| PVR (increase by 200 dyn·s·cm−5) | 1.34 (1.10–1.60) | 0.001 | |||
| CO (increase by 0.5 L/min) | 0.62 (0.48–0.82) | 0.001 | 0.61 (0.39–0.96) | 0.033 | |
| Time from diagnosis to surgery | 0.95 (0.93–1.04) | 0.644 | |||
| Residual PH | 1.25 (0.34–4.60) | 0.714 | |||
HR, hazard ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; PE, pulmonary embolism; WHO, World Health Organization; 6MWD, six-minute walk distance; RAP, right atrial pressure; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; CO, cardiac output; PH, pulmonary hypertension.
Discussion
CTEPH is a rare disease, with 8.9 cases per million inhabitants in Spain, despite it being thought to be underdiagnosed (1,2). Our study provides characteristics at diagnosis from 338 operable patients assessed in the CSUR expert centers for the management of complex PH, and outcomes after PEA including survival rates. Clinical guidelines (7) and consensus documents (1,2,17) establish the need to refer these patients to Multidisciplinary Expert Teams with expert surgeons to treat CTEPH. In fact, surgery should not be ruled out in any patient before being evaluated by an expert team. In the International CTPEH Registry (11), up to 43% of the patients evaluated were not considered candidates for surgery. A proportion of 75.7% of patients in the Spanish Registry (REHAP) did not undergo surgery (1). However, as previously shown, in CSUR specialized centers, almost 60% (58.5% of 578 patients) underwent surgery, after being evaluated by a Multidisciplinary Expert Team.
Analysis of the data revealed that the results of our series are excellent, despite severe previous hemodynamic condition. In our study population, 68.5% were classified as WHO III–IV functional class at the time of surgery (5.3% as WHO IV), and mPAP was 46.5±13.1 mmHg and mean PVR was 764.5±392.8 dyn·s·cm−5. Furthermore, PEA surgical times were similar to international specialized centers, including a mean total arrest time of 42.8±14.5 minutes and mean CBP of 215.8±47.0 minutes; despite concomitant surgery being performed in a group of 70 (20.6%) patients. Overall perioperative mortality was 3.3%, similar to the largest series reported in the literature by international specialized centers (8,17-19), and below mean European rates (7); placing Spanish CSUR CTEPH Centers in an outstanding international position. Our success is due to the expertise of our surgeons and the procedural protocol developed by the Multidisciplinary Expert Team. Surgical outcomes and survival in both centers in Madrid and Barcelona have improved as there has been a gradual increase in the number of surgical candidates and the team acquired more experience (2,20,21). The accessibility of lesions varies according to the level of experience of the surgical team. In most experienced teams, the percentage of patients with segmental branch involvement (San Diego type 3) increases over time, constituting 22.3% of total operated patients in CSUR centers (12,19). It is remarkable that our series registers very few cases of neurological complications (5.9%), most of them being transitory after PEA intervention, despite not performing PEA with continuous cerebral perfusion as suggested by other groups (22). Other postoperative complications were also rare, such as cardiac failure (6.5%), or ECMO implant, where only 2.1% of PEA patients required veno-arterial ECMO and 5.3% veno-venous ECMO. Reperfusion injury is inherent to PEA and its incidence ranges from 5% to 20%, as published by different series (10,12,14). According to our outcomes, reperfusion injury was recorded in 14.2% of patients. It is noteworthy that after PEA, hemodynamic and echocardiographic outcomes improved significantly; patients’ exercise capacity (6MWD), cardiac index and TAPSE index also improved; and NT-proBNP values decreased, as did mPAP and RAP values. Two important features must be highlighted; on one hand, PVR values decreased significantly (preoperative 757.8±375.2 vs. postoperative 329.0±477.5 dyn·s·cm−5, P<0.001), although 50% of patients had residual PH (mPAP >25 mmHg), while only 22.7% of patients had clinically relevant residual PH in RHC (PVR >400 dyn·s·cm−5) performed at six months after PEA, according to international registries (12,14,23). All such patients were individually assessed in order to choose further treatment options; 16.7% were candidates for BPA after surgery, and the rest were evaluated to receive targeted-MT such as riociguat, after its approval in Spain in 2015. Moreover, in our series we performed a six-month follow-up visit for two reasons: hemodynamic changes immediately following surgery will affect estimates of PVR, and also because the prevalence of residual PH increases over time when the cause is distal vascular disease (24).
In this study, potential factors associated with greater in-hospital mortality were also investigated. Previous PVR >1,000 dyn·s·cm−5 was a risk factor for in-hospital mortality in both the univariate and multivariable logistic regression analysis, similarly to other published series (12,19). Regarding follow-up, multiple mortality risk factors were found in the univariate Cox analysis. In the multivariable analysis, only longer preoperative distances in the six-minute walking test and high cardiac output were found to be independent protective factors for mortality, most likely due to better previous physical and hemodynamic conditions. Kaplan-Meier survival analysis showed outstanding survival rates of 94.8%, 93.3% and 90.5% in the one-, three- and five-year follow-up, respectively, similar to results reported in other major studies (12,15,19,23). For residual PH patients, survival rates were only slightly lower (94.8%, 87.8% and 87.8% at one-, three- and five-year follow-up, respectively) when compared with those patients whose PVR normalized after surgery (log-rank 0.004).
Excellent follow-up was achieved in 98.8% of patients; only 4 patients did not attend any check-up. A significant limitation when interpreting the results is that the data were collected by two different centers, where some data may be missing or inconclusive.
This study highlights the favorable results obtained in CSUR centers for CTEPH in Spain, with Multidisciplinary Expert Teams that evaluate patients according to their specific case and recommend the most appropriate treatment.
Conclusions
A cohort of 338 patients out of 578 patients diagnosed with CTEPH from 2007 to 2019, underwent PEA at two Spanish CTEPH specialized centers. Surgical patients had outstanding survival rates at one-, three- and five-year follow-up, and a high in-hospital survival rate for PEA patients was confirmed. Pulmonary endarterectomies were performed within short CBP and circulatory arrest times, with very few complications (including neurological, postoperative reperfusion edema, ECMO implant and cardiac failure) and good one-year results, where exercise capacity increased, and mPAP and PVR values significantly decreased. Mortality risk factors were also evaluated. Due to the optimal results obtained in CSUR centers, we reinforce our statement that all patients should be referred for operability assessment at specialized centers established by international guidelines.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martínez-Santos P, Velázquez-Martín MT, Barberá JA, et al. Chronic thromboembolic pulmonary hypertension in Spain: a decade of change. Rev Esp Cardiol (Engl Ed) 2021;74:384-92. [Crossref] [PubMed]
- López Gude MJ, Pérez de la Sota E, Forteza Gil A, et al. Pulmonary thromboendarterectomy in 106 patients with chronic thromboembolic pulmonary hypertension. Arch Bronconeumol 2015;51:502-8. [Crossref] [PubMed]
- McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007;93:1152-8. [Crossref] [PubMed]
- Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006;113:2011-20. [Crossref] [PubMed]
- Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008;177:1122-7. [Crossref] [PubMed]
- Escribano-Subias P, Blanco I, López-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012;40:596-603. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010;36:792-9. [Crossref] [PubMed]
- López Gude MJ, Pérez de la Sota E, Pérez Vela JL, et al. Pulmonary endarterectomy outputs in chronic thromboembolic pulmonary hypertension. Med Clin (Barc) 2017;149:1-8. [PubMed]
- Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160111. [Crossref] [PubMed]
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702-10. [Crossref] [PubMed]
- Madani MM. Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist Debakey Cardiovasc J 2016;12:213-8. [Crossref] [PubMed]
- Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319-29. [Crossref] [PubMed]
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124:1973-81. [Crossref] [PubMed]
- Rahnavardi M, Yan TD, Cao C, et al. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension : a systematic review. Ann Thorac Cardiovasc Surg 2011;17:435-45. [Crossref] [PubMed]
- Thistlethwaite PA, Mo M, Madani MM, et al. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2002;124:1203-11. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62:D92-9. [Crossref] [PubMed]
- Mayer E. Surgical treatment of chronic thromboembolic pulmonary hypertension. Swiss Med Wkly 2006;136:491-7. [PubMed]
- Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. [Crossref] [PubMed]
- Blázquez JA, Escribano P, Pérez E, et al. Chronic thromboembolic pulmonary hypertension: surgical treatment with thromboendarterectomy. Arch Bronconeumol 2009;45:496-501. [Crossref] [PubMed]
- Coronel ML, Chamorro N, Blanco I, et al. Medical and surgical management for chronic thromboembolic pulmonary hypertension: a single center experience. Arch Bronconeumol 2014;50:521-7. [Crossref] [PubMed]
- Vuylsteke A, Sharples L, Charman G, et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet 2011;378:1379-87. [Crossref] [PubMed]
- Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015;24:263-71. [Crossref] [PubMed]
- Corsico AG, D'Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med 2008;178:419-24. [Crossref] [PubMed]







