Surgical treatment of post-infarction papillary muscle rupture: systematic review and meta-analysis
Introduction
Papillary muscle rupture (PMR) is an uncommon, but often catastrophic complication of acute myocardial infarction (AMI), with recent literature reporting an incidence between 0.05% and 0.26% (1). PMR usually occurs within a week after AMI, especially as an evolution of inferior AMI (2). Despite mortality after surgical correction of PMR remarkably decreasing since the first successful mitral valve replacement (MVR) for PMR in 1965, the outcome of these subjects remains dismal (3). The poor results of medical treatment make surgical correction the standard of care for PMR (2). Although mitral valve repair (MVr) may lead to a better outcome due to greater preservation of post-operative left ventricular function, MVR is generally preferred in these high-risk patients (4). Since PMR is a rare event following AMI, most published series consist of single-center experiences with small sample sizes, and limited information regarding surgical results is available. We have therefore performed a systematic review and meta-analysis of the existing literature in order to provide a current perspective and summarize early post-operative outcomes and related predictors of the surgical correction of post-AMI PMR.
Methods
This systematic review and meta-analysis was registered with PROSPERO (ID: CRD42020163077) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (5).
Data sources, search strategy and selection criteria
PubMed, Embase and the Cochrane central register of controlled trials (CENTRAL) were screened for studies published from January 1, 1990 to the end of December 2020. The search terms were: “papillary muscle rupture” OR “mitral chordal rupture” OR “acute mitral regurgitation” OR “mechanical complication” AND “myocardial infarction” OR “surgical treatment”. The literature was limited to articles published in English. Studies which provided the outcomes for adult patients (>18 years old) who underwent surgical correction of post-AMI PMR were included. Articles were excluded if they included: (I) animal studies; (II) PMR not AMI-related (e.g., post-traumatic); (III) studies including <20 surgical patients; (IV) duplicate publications from the same center reporting overlapping patient data. Case reports and systematic reviews were not considered. Reference lists were reviewed manually and cross-checked for other relevant reports.
Data extraction and endpoint selection
Two independent reviewers (G Massimi and M Matteucci) selected the studies for inclusion and extracted articles, as well as patient characteristics of interest and relevant outcomes. A standardised form was used to extract data of interest. Discrepancies were resolved by discussion and adjudication by a third reviewer (R Lorusso). The primary outcome being assessed was operative mortality, defined as any death, regardless of cause, occurring within 30 days after surgery (in or out of hospital) or after 30 days but during the index hospitalization subsequent to the surgery. Secondary endpoints were the following in-hospital postoperative complications: stroke, major bleeding, renal failure requiring renal replacement therapy (RRT), and low cardiac output syndrome (LCOS). Long-term follow-up and out-of-hospital complications were not considered.
Quality assessment
Two authors (G Massimi and M Matteucci) independently assessed the trials’ eligibility and risk of bias. Risk of bias at the individual study level was appraised with ROBINS-I (Risk Of Bias In Not-randomized Studies of Interventions), a tool used for assessment of bias (the selection of the study groups; the comparability of the groups; the ascertainment of either the exposure or outcome of interest) in cohort studies included in a systematic review and/or meta-analysis (6). Any divergences were resolved by a third reviewer (R Lorusso).
Statistical analysis
Pooled risks ratios (RRs) were reported with 95% confidence intervals (CIs). The Cochran’s Q test and I2 test were all performed to judge the heterogeneity among the studies included in the meta-analysis. Heterogeneity was considered to be significant at P<0.1 for the Q statistic. An I2 value of less than 50% indicates low heterogeneity, values between 50% and 75% suggest moderate heterogeneity, and I2 greater than 75% was considered high heterogeneity. Sensitivity analysis was carried out by successively excluding the low-quality studies to assess the stability of the outcome. Potential publication bias was evaluated by constructing a funnel plot. The plot was estimated visually, and asymmetry in the funnel plot suggested possible publication bias. Review Manager 5.3 software, developed by the Cochrane Collaboration (http://tech.cochrane.org/revman/), was used for statistical computations. A value of P<0.05 was considered statistically significant.
Results
We identified 3,023 reports, reviewed 62 full text articles, and identified 12 studies that met explicit inclusion criteria (4,7-17), enrolling a total of 1,851 patients. Of the 12 articles included, all were observational and retrospective in design. Ten studies were considered to have adequate criteria to be included in the meta-analysis. The PRISMA flow chart depicting the study selection process is presented as Figure S1.
Risk of bias
A summary of the risk of biases of included studies is reported in Table S1. Overall, quality assessment revealed a significant risk of bias, in particular due to confounding and selection bias. Analysis of the funnel plots showed symmetry and suggested no significant risk of publication bias or big/small study effect (Figures S2,S3).
Baseline and operative characteristics
Mean age of the patients was 66±4 years, and men accounted for 67% of cases. All subjects had acute severe mitral regurgitation caused by post-infarction PMR. The rate of individuals in cardiogenic shock was 59% (909/1,679 patients) at the time of operation, with 44% (634 patients) requiring inotropic support. Severe left ventricular systolic dysfunction (LVEF <30%) was present in one third of the patients (531/1,608). Pre- or perioperative IABP was inserted in almost 60% of subjects. Detailed characteristics of studies and patients are listed in Table 1. The rupture involved the postero-medial papillary muscle in 77% (182/235) of cases, and the rupture was partial or incomplete (head rupture) in 54% of subjects. Patients most commonly underwent MVR; MVr was performed in only 18% (319/1,792) of cases. Mean duration of cardiopulmonary bypass (CPB) was 147±31 minutes. Fifty-seven percent of patients had concomitant coronary artery bypass grafting (CABG) at the time of mitral valve surgery. Postoperative IABP support was necessary in almost two-thirds of the patients. Operative data is shown in Table 2.
Table 1
| Author (ref.) | Year of publication | Study period | Country | Patients (n) |
Age* (years) | Male (n) | Shock (n) | Inotropes (n) | IABP (n) |
|---|---|---|---|---|---|---|---|---|---|
| Fujita (14) | 2020 | 2014–2017 | Japan | 196 | 74 | 119 | 140 | – | 159 |
| Kilic (15) | 2020 | 2011–2018 | USA | 1,342 | 66 | 911 | 759 | 582 | 764 |
| Sultan (11) | 2018 | 2011–2017 | USA | 24 | 62 | 15 | – | – | 14 |
| Ternus (7) | 2017 | 2000–2014 | USA | 22 | 70 | 16 | 15 | – | 15 |
| Bouma (9) | 2014 | 1990–2012 | The Netherlands | 48 | 65 | 34 | 31 | 26 | 21 |
| Schroeter (13) | 2013 | 2002–2010 | Germany | 28 | 63 | 22 | 15 | – | 12 |
| Russo (16) | 2008 | 1980–2000 | USA | 54 | 70 | 40 | – | – | – |
| Chevalier (10) | 2004 | 1985–2002 | France | 37 | – | – | – | – | – |
| Chen (17) | 2002 | 1978–2000 | UK | 33 | 64 | 20 | – | 26 | 17 |
| Tavakoli (4) | 2002 | 1988–1998 | Switzerland | 21 | 62 | – | 21 | – | 11 |
| Figueras (8) | 1997 | 1979–1995 | Spain | 24 | – | – | – | – | – |
| Kishon (12) | 1992 | 1981–1990 | USA | 22 | 68 | 15 | 15 | – | 13 |
| Total [%] or (± SD) | – | – | – | 1,851 | 66 (±4) | 1,192 [67] | 996 [59] | 634 [44] | 1,026 [59] |
*, mean value. Ref., reference; n, number; y, years; IABP, intra-aortic balloon pump; SD, standard deviation.
Table 2
| Author (ref.) | A-L PMR (n) | P-M PMR (n) | Head rupture (n) | Body rupture (n) | MVR (n) | MVr (n) | CPB* time (m) | Conc. CABG (n) | IABP (n) |
|---|---|---|---|---|---|---|---|---|---|
| Fujita (14) | – | – | – | – | 176 | 20 | 156 | 60 | – |
| Kilic (15) | – | – | – | – | 1,071 | 271 | 162 | 796 | – |
| Sultan (11) | 6 | 18 | 15 | 9 | 17 | 7 | 171 | 13 | – |
| Ternus (7) | 12 | 10 | 12 | 10 | – | – | – | – | – |
| Bouma (9) | 5 | 42 | 28 | 20 | 38 | 10 | 178 | 24 | 24 |
| Schroeter (13) | 11 | 11 | – | – | 25 | 3 | 151 | 19 | 20 |
| Russo (16) | 6 | 48 | – | – | 41 | 13 | 89 | 42 | 39 |
| Chevalier (10) | 6 | 31 | 12 | 25 | – | – | – | – | – |
| Chen (17) | – | – | – | – | 31 | 2 | – | 20 | 21 |
| Tavakoli (4) | – | – | – | – | 19 | 2 | – | 19 | – |
| Figueras (8) | – | – | – | – | 24 | 0 | – | 8 | – |
| Kishon (12) | 0 | 22 | 15 | 7 | 21 | 1 | 121 | 17 | – |
| Total [%] or (± SD) | 46^ [20] | 182 [77] | 82 [54] | 71 [46] | 1,463 [82] | 329 [18] | 147 (±31) | 1,018 [57] | 104 [64] |
*, mean value; ^, in the remining 3% of cases, the rupture involved both A-L and P-M papillary muscles. Ref., reference; A-L, antero-lateral; P-M, postero-medial; PMR, papillary muscle rupture; n, number; MVR, mitral valve replacement; MVr, mitral valve repair; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; Conc., concomitant; IABP, intra-aortic balloon pump; m, minutes; SD, standard deviation.
Postoperative outcomes
Overall, the total number of early deaths was 392, representing an operative mortality rate of 21%. Post-operatively, kidney dysfunction requiring RRT occurred in 13% of subjects, whereas major bleeding requiring re-intervention and stroke occurred in 16% and 5% of cases, respectively. Mean hospital length of stay was 18.9±11.3 days. The most common cause of postoperative death was LCOS (45%). Surgical outcomes are outlined in Table 3.
Table 3
| Author (ref.) | Stroke (n) | RRT (n) | Major bleeding (n) | LCOS (n) |
H stay* (d) |
Operative mortality (n) | Cardiac cause (n) |
Other cause (n) |
|---|---|---|---|---|---|---|---|---|
| Fujita (14) | 16 | 35 | – | – | 28 | 50 | – | – |
| Kilic (15) | 70 | 161 | – | – | 16 | 268 | – | – |
| Sultan (11) | 0 | 2 | 2 | – | 19 | 3 | 3 | 0 |
| Ternus (7) | – | – | – | – | – | 2 | 2 | 0 |
| Bouma (9) | 0 | 7 | 8 | – | 19 | 12 | 10 | 2 |
| Schroeter (13) | – | 16 | 6^ | 16 | 8 | 11 | – | – |
| Russo (16) | – | – | – | 16 | 20 | 10 | 8 | 2 |
| Chevalier (10) | – | – | – | – | – | 8 | – | – |
| Chen (17) | – | – | – | – | – | 7 | – | – |
| Tavakoli (4) | 1 | 3 | – | – | – | 4 | 2 | 2 |
| Figueras (8) | – | – | – | – | – | 11 | 4 | 7 |
| Kishon (12) | – | – | – | – | – | 6 | 4 | 2 |
| Total [%] or (± SD) | 87 [5] | 224 [13] | 16 [16] | 32 [39] | 18 (±7) | 392 [21] | 33 [69] | 15 [31] |
*, mean value; ^, re-thoracotomy for unclear reason. Ref., reference; n, number; RRT, renal replacement therapy; LCOS, low cardiac output syndrome; H, hospital; d, days; SD, standard deviation.
Operative mortality
Operative mortality was significantly increased in patients with complete (body rupture) PMR as compared to partial or incomplete PMR (head rupture) (RR, 2.54; 95% CI: 1.12 to 5.74; P=0.03; I2=0%) (Figure 1), with early death rates of 31.5% (17/54) and 10.9% (6/55), respectively. Subjects undergoing MVr had a reduced risk of operative mortality (RR, 0.33; 95% CI: 0.14 to 0.79; P=0.01; I2=53%) (Figure 2) as compared to those undergoing MVR. Operative mortality rate was 24.3% (322/1,326) and 5.7% (18/314) for MVR and MVr respectively. There was no significant difference in the risk of operative mortality between patients with or without pre/peri-operative IABP support (RR, 2.62; 95% CI: 0.56 to 12.17; P=0.22) and between subjects undergoing mitral valve surgery with or without concomitant CABG (RR, 0.61; 95% CI: 0.36 to 1.06; P=0.08), with moderate heterogeneity among studies (I2=54% and I2=64%, respectively) (Figures 3,4). Mortality rates were 20% (182/906) in patients with concomitant CABG, 22.2% (136/612) in no-CABG patients, and 35.3% (18/51) and 14.9% (7/47) for pre/peri-operative IABP support versus no-IABP respectively.

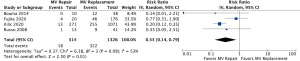

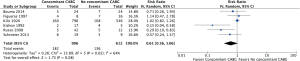
Sensitivity analysis
Analysis performed by successively deleting the studies at highest risk of bias did not reveal any change in direction nor magnitude of the treatment effect.
Discussion
PMR is a rare but serious mechanical complication of AMI. It occurs in less than 1% of patients sustaining AMI (1), and accounts for 5% of infarct-related deaths (1). Most ruptures develop within seven days after AMI, but a delayed rupture may also occur (2). Post-AMI PMR is usually characterized by pulmonary edema and cardiogenic shock, ultimately leading to multiorgan failure and death (18). Early diagnosis and prompt management are therefore paramount to ensure successful treatment and patient survival. Immediate surgical correction is considered the optimal and most rational treatment for acute post-AMI PMR; however, even for patients who are treated surgically, mortality is high, ranging between 9% and 45% (7,8). Real-world results in the modern era indicate that no major improvements have been observed in the last two decades (18), highlighting that careful evaluation is required to understand potential areas for improvement. This systematic review provides an overview of published evidence on the characteristics and outcomes after surgical treatment of post-AMI PMR.
Posteromedial PMR is far more common than anterolateral PMR (2) given the coronary anatomy and arterial blood supply of the papillary muscles (3). Indeed, the anterolateral papillary muscle has a dual blood supply from the left anterior descending and left circumflex coronary arteries, whereas the posteromedial papillary muscle has a single blood supply from the posterior descending artery (2). In this review, ruptures involved mostly the posterior papillary muscle (77%), which supports previous observations.
PMR may be complete (usually occurring at the base of the papillary muscle) or partial occurring at one of the tips (heads) of a papillary muscle. Partial PMR can lead to varying degrees of mitral insufficiency, whereas complete PMR causes prolapse of both the anterior and posterior leaflet and subsequently, severe mitral regurgitation. Our results showed a significantly higher frequency of operative mortality in complete PMR (body rupture) than partial PMR. While partial rupture occurred slightly more frequently than complete rupture, patients were more likely to have worse conditions and preoperative hemodynamic instability in complete PMR, which is also consistent with the literature (9-11).
Despite alternative approaches, such as MitraClip, having been increasingly proposed to treat patients with post-AMI PMR (19,20), retrospective studies have shown that the in-hospital mortality rate in patients undergoing surgery is remarkably better (21), mainly in subjects with pre-operative hemodynamic instability and cardiogenic shock. The operative mortality in the current study was relatively low, when compared to the early mortality of patients undergoing surgery for other post-infarction mechanical complications, such as ventricular septal rupture (VSR) (22) (21% versus 38%, respectively).
PMR can be addressed with either MVr in select patients, or MVR. When post-infarction PMR is complete, repair is often not feasible because of necrotic and friable infarcted tissue. Mitral regurgitation secondary to partial or incomplete PMR, with limited adjacent tissue damage, is often amenable to a reliable and durable repair. In this review, MVR was carried out in almost 85% of cases. Moreover, we observed a higher operative mortality rate after MVR. A possible explanation for this is the critical illness status of patients in whom MVR was undertaken. MVR is usually reserved for subjects with complete PMR or partial/incomplete PMR and compromised hemodynamic stability at surgery in order to reduce CPB, ischemic times, and related risks (10-12).
The impact of concomitant CABG on the outcomes of patients undergoing cardiac surgery for post-AMI mechanical complication remains unclear. In our previous meta-analysis, we did not find any significant protective effect of simultaneous CABG in addition to septal defect repair in the setting of post-AMI VSR (22). Similarly, in this study, concomitant CABG did not influence early survival. Further analysis is needed to determine the importance of simultaneous CABG in the context of mitral valve surgery for post-AMI PMR.
Pre-operative hemodynamic instability and cardiogenic shock are frequent scenarios after the occurrence of post-AMI PMR, making the use of IABP effective and generally accepted by current guidelines (23). Such an approach may therefore be helpful in improving the hemodynamic stability of patients and allow postponement of surgical intervention. In the current review, IABP was used in almost half of PMR patients prior to surgery. However, similar to the findings reported in several other studies (3,9,13), our analysis showed no significant difference in the risk of operative mortality between patients with or without pre/peri-operative IABP support. A possible explanation for this is the critical illness status of patients in whom the decision of IABP insertion was made.
In patients with extremely or very compromised pre-operative hemodynamic stability, more aggressive mechanical circulatory support (MCS), such as extracorporeal membrane oxygenation (ECMO), has been shown to be a useful strategy for the treatment of post-AMI PMR in the setting of univentricular or biventricular failure, either preoperatively as a bridge to surgery, or postoperatively following mitral valve operation (24). ECMO allows circulatory support, providing time and hemodynamic stability for diagnostic workup and surgical intervention planning, while reversing organ damage. This improvement occurs at the expense of a high rate of device-related complications (25), so patient selection and the single center’s experience are important to achieve satisfactory results. The literature relating to the use of ECMO in the context of PMR is limited to successful case reports and as part of small observational studies (13-15) depicting the utility of ECMO as a way of stabilizing inoperable or high-mortality surgical candidates. However, the lack of specific information in the ECMO subgroup prevented us from exploring this issue.
Limitations
The retrospective nature of the reports included represents the major limitation of this review. Retrospective studies are subject to confounder bias, possibly affecting the conclusive power of our meta-analysis. The pooled occurrence rates for complications and mortality were based on heterogeneous data and should be treated with considerable reserve. Individual and institutional experience is crucial in determining the likelihood of the success of PMR surgery. This is therefore an important current subgroup analysis, limited by the number of included studies, and should be viewed with caution. Better results observed with mitral valve repair and concomitant CABG may not be reflected in centres experienced with early mitral valve replacement and high-risk percutaneous coronary interventions (PCIs). Although our analysis revealed no evidence of significant reporting bias, such bias still remains a possibility, with potentially more favourable results being reported from large-volume expert centres that may not be representative of all institutions. Trends in characteristics and outcomes in this review are largely driven by the Society of Thoracic Surgeons (STS) registry (15); the value in this study is that the more geographically diverse data presented here does not substantially differ in findings from the large STS dataset published by Kilic et al. Two national registries provided data for this review (14,15), with the potential risk of patients overlapping accounting for less than 2.5% of the population (7,11). As the timeline of the study period is fairly long, progress in management and operative strategies may have changed over time, limiting our qualitative analysis. Another important limitation of the current review is the considerable amount of missing data. Finally, because this study is limited to operative outcomes, it does not provide information on the durability of surgical PMR procedures.
Conclusions
Mitral valve surgery for post-AMI PMR is associated with high operative mortality (21%). The findings of the present meta-analysis seem to indicate that the risk of operative mortality is higher in the presence of complete PMR and in subjects undergoing MVR. Our results also suggest that concomitant CABG during PMR correction and the pre/peri-operative use of IABP do not improve early survival. More aggressive pre- or post-operative MCS use might presumably be of some help in unstable patients with a high risk of surgery, in an attempt to improve outcomes. However, more data and studies are warranted to conclusively indicate the actual potential and role of such an approach in post-AMI PMR.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Prof. RL is a a consultant for Medtronic, Getinge and LivaNova, and Member of the advisory board of Eurosets and Fresenius/Xenios. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhardwaj B, Sidhu G, Balla S, et al. Outcomes and Hospital Utilization in Patients With Papillary Muscle Rupture Associated With Acute Myocardial Infarction. Am J Cardiol 2020;125:1020-5. [Crossref] [PubMed]
- Nishimura RA, Schaff HV, Shub C, et al. Papillary muscle rupture complicating acute myocardial infarction: analysis of 17 patients. Am J Cardiol 1983;51:373-7. [Crossref] [PubMed]
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- Tavakoli R, Weber A, Vogt P, et al. Surgical management of acute mitral valve regurgitation due to post-infarction papillary muscle rupture. J Heart Valve Dis 2002;11:20-5; discussion 26. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Ternus BW, Mankad S, Edwards WD, et al. Clinical presentation and echocardiographic diagnosis of postinfarction papillary muscle rupture: A review of 22 cases. Echocardiography 2017;34:973-7. [Crossref] [PubMed]
- Figueras J, Calvo F, Cortadellas J, et al. Comparison of patients with and without papillary muscle rupture during acute myocardial infarction. Am J Cardiol 1997;80:625-7. [Crossref] [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Koene BM, et al. Predictors of in-hospital mortality after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg 2014;9:171. [Crossref] [PubMed]
- Chevalier P, Burri H, Fahrat F, et al. Perioperative outcome and long-term survival of surgery for acute post-infarction mitral regurgitation. Eur J Cardiothorac Surg 2004;26:330-5. [Crossref] [PubMed]
- Sultan I, Aranda-Michel E, Gleason TG, et al. Mitral valve surgery for acute papillary muscle rupture. J Card Surg 2018;33:484-8. [Crossref] [PubMed]
- Kishon Y, Oh JK, Schaff HV, et al. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc 1992;67:1023-30. [Crossref] [PubMed]
- Schroeter T, Lehmann S, Misfeld M, et al. Clinical outcome after mitral valve surgery due to ischemic papillary muscle rupture. Ann Thorac Surg 2013;95:820-4. [Crossref] [PubMed]
- Fujita T, Yamamoto H, Kobayashi J, et al. Mitral valve surgery for ischemic papillary muscle rupture: outcomes from the Japan cardiovascular surgery database. Gen Thorac Cardiovasc Surg 2020;68:1439-46. [Crossref] [PubMed]
- Kilic A, Sultan I, Chu D, et al. Mitral Valve Surgery for Papillary Muscle Rupture: Outcomes in 1342 Patients From The Society of Thoracic Surgeons Database. Ann Thorac Surg 2020;110:1975-81. [Crossref] [PubMed]
- Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation 2008;118:1528-34. [Crossref] [PubMed]
- Chen Q, Darlymple-Hay MJ, Alexiou C, et al. Mitral valve surgery for acute papillary muscle rupture following myocardial infarction. J Heart Valve Dis 2002;11:27-31. [PubMed]
- Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82. [Crossref] [PubMed]
- Alajaji WA, Akl EA, Farha A, et al. Surgical versus medical management of patients with acute ischemic mitral regurgitation: a systematic review. BMC Res Notes 2015;8:712. [Crossref] [PubMed]
- Valle JA, Miyasaka RL, Carroll JD. Acute Mitral Regurgitation Secondary to Papillary Muscle Tear: Is Transcatheter Edge-to-Edge Mitral Valve Repair a New Paradigm? Circ Cardiovasc Interv 2017;10:e005050. [Crossref] [PubMed]
- Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36:1104-9. [Crossref] [PubMed]
- Matteucci M, Ronco D, Corazzari C, et al. Surgical Repair of Postinfarction Ventricular Septal Rupture: Systematic Review and Meta-Analysis. Ann Thorac Surg 2021;112:326-37. [Crossref] [PubMed]
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4-90. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. The use of extracorporeal membrane oxygenation in the setting of postinfarction mechanical complications: outcome analysis of the Extracorporeal Life Support Organization Registry. Interact Cardiovasc Thorac Surg 2020;31:369-74. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]






