Triple patch technique to repair ventricular septal rupture
Introduction
Postinfarction ventricular septal rupture (VSR) is a rare, but life-threatening complication of myocardial infarction (MI). The incidence of postinfarction VSR is decreasing with the advent of reperfusion therapy. It has been reported that the prevalence of postinfarction VSR is 0.17% to 0.31% of acute MI cases (1,2).
Despite the development of numerous improvements in surgical techniques and materials, the mortality rate associated with postinfarction VSR remains high. Indeed, according to contemporary and systematic reviews, the operative mortality rates are 38.2% and 42.9%, respectively (1,3).
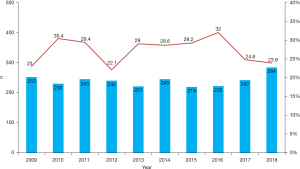
According to annual reports from the Japanese Association for Thoracic Surgery, 650 VSR repairs were performed between 2009 and 2018 (4-13). The 30-day mortality rates were between 22.1% and 32.0% (Figure 1). The outcomes have not improved as much as we had expected. In addition, it is well known that a residual shunt may affect cardiac function or late outcomes after a VSR repair. The reported incidence of residual shunt rates after VSR repair ranges from 23–43% (14,15).
Since the first surgical case report, many procedures have been proposed to decrease postoperative residual shunts. Among them, the infarct exclusion repair described by David and Komeda has become a popular procedure (16). Recently, the sandwich technique via a right ventricle incision has been reported with relatively good outcomes (17,18). In 2004 we began treating postinfarction VSR using the triple patch technique, which is a modified infarct exclusion method (19,20). Before 2004, we performed VSR repairs using the original infarct exclusion method; however, postoperative residual shunts were present in 50% of cases. We assumed that this problem might depend on the technical complexity of the procedure. Therefore, we modified the conventional infarct exclusion technique to easily determine the size and shape of the left ventricular pouch, and to make it simpler to suture the patch to the myocardium.
Triple patch technique
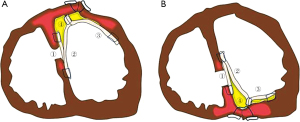
A median sternotomy is performed under general anesthesia. Cardiopulmonary bypass is initiated using ascending aorta and bicaval cannulation under mild hypothermia. Coronary artery bypass grafting (CABG), if necessary, is performed on the beating heart before the VSR repair. Both antegrade and retrograde cold blood cardioplegia provide myocardial protection. The operative scheme is shown in Figure 2. A VSR repair is performed through a longitudinal left ventriculotomy in the infarcted area, approximately 2 cm away from the left anterior descending coronary artery (anterior type) or the posterior descending artery (posterior type), depending on the site of MI.
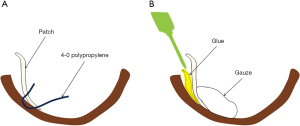
First, a tailored bovine pericardial patch (1st patch) is used to close the ventricular septal defect (VSD) directly with a running 4-0 polypropylene suture. The first patch is also used to prevent glue from entering the right ventricle. Then, two bovine pericardial patches are cut into adequate sizes. One pericardial patch (2nd patch) is sutured to the non-infarcted endocardium around the ventricular septal side with a 4-0 polypropylene over and over suture, and the other patch (3rd patch) is sutured to the non-infarcted endocardium on the ventricular free wall.
With respect to the 2nd and 3rd patches, the cranial margin of the patch is fixed to the myocardium with a 4-0 polypropylene mattress suture brought inside the ventricular free wall. The ends of the running sutures are brought outside the ventricular free wall at the apical side and tied.
After suturing, Bioglue (CryoLife Inc., Kennesaw, GA, USA) is applied to the suture line area on the muscle side. These two patches are then cut and sewn to determine the ideal size and shape of the pouch fitting the left ventricular cavity. These patches are sutured with 4-0 polypropylene over and over running sutures.
Finally, glue is applied to fill the cavity between the first patch and the pouch, and the ventriculotomy is closed with two teflon felt strips and 2-0 polypropylene mattress sutures.
Outcomes
Between April 2004 and December 2020, VSR repairs using the triple patch technique were performed in 42 patients at our institution. Patient demographics, clinical characteristics and early outcomes are summarized in Table 1. The mean age was 74.6±9.2 (range, 49–90) years. Twelve patients (28.6%) were in cardiogenic shock at the time of surgery. An intra-aortic balloon pump (IABP) was used in all patients preoperatively.
Table 1
| Variables | N (%)/mean ± SD |
|---|---|
| Age (years) | 74.6±9.2 |
| Range | 49–90 |
| Shock status | 12 (28.6) |
| Preoperative IABP | 42 (100.0) |
| Preoperative ECMO | 4 (9.5) |
| Preoperative PCI | 7 (16.7) |
| Urgent or emergent status | 23 (54.8) |
| Duration from MI to operation (days) | 9.2±8.4 |
| Median (days) | 7 |
| EF (%) | 45.8±14.0 |
| <40% | 18 (42.9) |
| Qp/Qs | 2.78±1.05 |
| Oozing rupture | 1 (2.4) |
| Aortic cross-clamp time (min) | 99±31 |
| VSD | |
| Anterior | 30 (71.4) |
| Posterior | 12 (28.6) |
| Concomitant procedure | |
| CABG | 11 (26.2) |
| Distal anastomosis | 1.2±0.4 |
| MVR | 3 (7.2) |
| TAP | 1 (2.4) |
| PM implantation | 1 (2.4) |
| Postoperative ECMO | 5 (12.0) |
| Permanent stroke | 2 (4.8) |
| Re-operation | 0 (0.0) |
| 30-day mortality | 9 (21.4) |
| Posterior type | 3 |
| Residual shunt | 6 (14.3) |
| Posterior type | 0 (0.0) |
| Qp/Qs | 1.47±0.32 |
SD, standard deviation; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; PCI, percutaneous coronary intervention; MI, myocardial infarction; EF, ejection fraction; Qp/Qs, pulmonary/systemic blood flow ratio; VSD, ventricular septal defect; CABG, coronary artery bypass grafting; MVR, mitral valve replacement; TAP, tricuspid annuloplasty; PM, pacemaker.
Additionally, preoperative extracorporeal membrane oxygenation (ECMO) was required in 4 patients (9.5%). The mean and median times from MI to surgery were 9.2±8.4 and 7 days, respectively. Eighteen patients (42.9%) had a left ventricular ejection fraction (EF) ≤40%. The mean pulmonary/systemic blood flow ratio (Qp/Qs) was 2.78±1.05 (range, 1.4–5.27). Twenty-three patients (54.8%) underwent urgent or emergent surgery.
The mean aortic cross clamp (ACC) time was 99±31 (range, 58–188) minutes, respectively. Anterior and posterior VSRs were repaired in 30 (71.4%) and 12 patients (28.6%), respectively. A concomitant CABG was performed in 11 patients (26.2%).
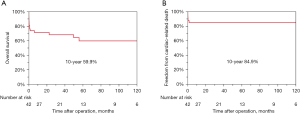
The 30-day mortality rate was 21.4%. Eight of these patients were in cardiogenic shock preoperatively, three of whom had a posterior VSR. The incidence of permanent stroke was 2 (4.8%). Six patients (14.3%) had a residual shunt after VSR repair. The overall 10-year survival rate was 59.9%, and the overall freedom from cardiac-related death at 10 years was 84.9% (Figure 3). There were significant differences in surgical outcomes between patients who underwent anterior and posterior VSR repairs.
Patients who died during the first 30 days
Nine patients died within 30 days after surgery. The preoperative mean EF of these patients was 38.6%±14.4%. Eight of the patients were in cardiogenic shock preoperatively; emergent operation was performed in six of the patients. One patient had a left ventricular free wall rupture (oozing type). Eventually, five of nine patients died of low output syndrome (LOS), three died of sepsis and one died of stroke. There were four patients who required postoperative veno-arterial (V-A) ECMO. Unfortunately, none of the patients could be withdrawn from V-A ECMO and died of LOS.
Prognosis of patients with a residual shunt after VSR repair
Six patients (14.3%) had a slight residual shunt after VSR repair (Table 2). All of the patients had an anterior type rupture. Emergent operation was performed in four patients. The mean and median Qp/Qs of the six patients were 1.47±0.32 and 1.35, respectively. One of six patients died on postoperative day 46 due to LOS; the postoperative Qp/Qs was 2.0. The other patients were discharged.
Table 2
| Case | VSD | Postoperative Qp/Qs | Follow up Qp/Qs | Alive (year since surgery) |
|---|---|---|---|---|
| Case 1 | Anterior | 2.0 | – | No (in-hospital death) |
| Case 2 | Anterior | 1.5 | 1.5 | No (6 years) |
| Case 3 | Anterior | 1.7 | 1.5 | Yes (12 years) |
| Case 4 | Anterior | 1.6 | 1.0 | Yes (7 years) |
| Case 5 | Anterior | 1.5 | 1.3 | Yes (3.7 years) |
| Case 6 | Anterior | 1.2 | 1.2 | Yes (2.6 years) |
VSD, ventricular septal defect; Qp/Qs, pulmonary/systemic blood flow ratio.
During the follow up period, the residual shunt disappeared 6 months after VSR repair in one patient, gradually decreased in two and remained unchanged in one. A patient with a postoperative Qp/Qs of 1.5 died 5 years after surgery due to non-cardiac disease. The residual shunt flow did not worsen over time.
Comments
Patch fixation and the use of glue
An ideal suture line may be difficult to choose in the acute setting. Therefore, the patch should be gently and softly placed with an over and over running suture to avoid overtightening or myocardial tearing. In addition, the suture should be inserted from patch to patch through the myocardium (Figure 4A). Using this suture method, the patch can be fixed securely with minimal shifting from the position. It is acceptable if the running suture is slightly loosened, because the patch can be firmly attached to the endocardium using glue. Reinforcement using glue is also one of the important options for the VSR repair. Fibrin glue or Bioglue are used at our institution. Recently, we began routinely using Bioglue. Some authors have reported that the glue can reduce the residual shunt and the wide adhesion can prevent excessive pressure on the suture line (21,22). Moreover, Komeda reported that BioGlue renders the fragile myocardium stiffer and easier to suture (23). We also believe that the use of glue is ideal to secure patch fixation, even though the myocardium is infarcted or weakened. It is also important that gauze is placed inside the left ventricle before using glue to prevent dripping glue into the left ventricle (Figure 4B).
In addition, no bleeding from the left ventriculotomy closure occurred in our series, which may be due to the effectiveness of the glue applied in the dead space or around the ventriculotomy.
Posterior VSR
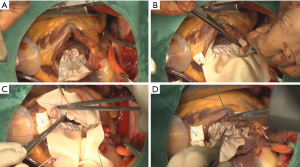
It has been suggested that surgical repair of a posterior VSR carries higher rates of mortality and morbidity than does repair of an anterior VSR (15,24). Posterior VSR repairs are commonly associated with right ventricular dysfunction or technical difficulties in exposure and suturing; however, the triple patch technique can be performed without difficulty, even for a posterior VSR (Figure 5). In the case of a posterior type, generally one pericardial patch is sutured and fixed to the fibrous annulus of the mitral valve. The other patch is sutured to the posterior wall along a line of the medial margin at the base of the posteromedial papillary muscle. We can clearly see the VSR site, mitral valve or mitral subvalvular apparatus, and both an anterior and a posterior VSR repair can be easily performed. Indeed, there were no residual shunts in patients who underwent a posterior VSR repair, and there was no significant difference in surgical outcomes between anterior and posterior VSRs in our study.
Concomitant CABG
It is controversial whether concomitant CABG improves outcomes after a VSR repair. Some authors have reported that no or incomplete revascularization is an independent predictor of mortality and that complete revascularization improves long-term survival (25,26).
In contrast, in a systematic review and meta-analysis of surgical repairs of postinfarction VSR, Matteucci et al. reported that there was no significant protective effect with simultaneous CABG in terms of the early mortality rate (3). Sakaguchi et al. argued that revascularization of transmurally infarcted myocardium is illogical because the culprit artery is often entrapped in the suture line of the ventriculotomy, which can make bypass impossible (27). We agree, for the most part, with their opinion. In addition, most of the culprit arteries were completely occluded in VSR patients based on our experiences. Therefore, we generally perform CABG for other stenotic coronary arteries, but not the culprit artery. Indeed, no cardiac events related to the culprit artery for VSR occurred during our follow up period. In our series, percutaneous coronary intervention (PCI) for culprit lesions was performed in seven patients with acute coronary syndrome before diagnosing postinfarction VSR.
LOS
In the case of hemodynamic instability, mechanical circulatory support is often required, which can complicate treatment due to organ dysfunction. As pre- or post-operative management, we commonly use IABP in VSR patients and V-A ECMO in hemodynamically unstable patients. In terms of survival, the influence of preoperative organ dysfunction is also considerable, as previously reported (20). We also recognize that the poor outcome is related to extensive MI.
Daubert et al. reported that cardiac remodeling after an extensive MI gradually occurs and that 46% of all patients demonstrated reverse remodeling at 1 month (28). Among patients with extensive MI, it takes a long time to recover from myocardial damage or for cardiac remodeling to occur. Thus, an extensive MI appears to have a huge impact on survival, even though there is no residual shunt after the VSR repair.
One of the mechanical circulatory supports, Impella (Abiomed, Danvers, MA, USA) may be an alternative device for the treatment of VSR, especially in patients with LOS, although we have no experience in the use of Impella. Some authors have reported that the Impella can improve surgical outcome for postinfarction VSR (29-32). Impella has been shown to effectively reduce afterload and decrease flow through the VSD, thus leading to an increase the in mean arterial pressure and a decrease in the pulmonary capillary wedge pressure. It has also been reported that Impella’s effect of reducing the left ventricular load is superior to that of IABP (33). The purpose of Impella is to achieve recovery from myocardial damage by assisting antegrade blood flow and reducing the load on the ventricle. As a result, the use of Impella may reduce organ damage compared to ECMO alone. Moreover, Via et al. reported that Impella allows bridging to appropriately timed surgical repair (34). Therefore, Impella may change the surgical strategy for postinfarction VSR before and after repair, although Imeplla also has several problems, such as bleeding or hemolysis. We hope that Impella will contribute to improved prognosis among patients with postinfarction VSR.
Conclusions
The early and late outcomes of modified infarct exclusion using the triple patch technique are acceptable. This technique is safe and simple, and may be useful for reducing postoperative residual shunts.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J 2014;35:2060-8. [Crossref] [PubMed]
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- Matteucci M, Ronco D, Corazzari C, et al. Surgical repair of post-infarction ventricular septal rupture: systematic review and meta-analysis. Ann Thorac Surg 2021;112:326-37. [Crossref] [PubMed]
- Committee for Scientific Affairs. Thoracic and cardiovascular surgery in Japan during 2009: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2011;59:636-67. [Crossref] [PubMed]
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010 : annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012;60:680-708. [Crossref] [PubMed]
- Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2013;61:578-607. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2012 : annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734-64. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2014 : Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665-97. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan during 2015 : Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2018;66:581-615. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. Erratum in: Gen Thorac Cardiovasc Surg 2019;67:573-75. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2018: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2021;69:179-212. [Crossref] [PubMed]
- Deja MA, Szostek J, Widenka K, et al. Post infarction ventricular septal defect - can we do better? Eur J Cardiothorac Surg 2000;18:194-201. [Crossref] [PubMed]
- Jeppsson A, Liden H, Johnsson P, et al. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg 2005;27:216-21. [Crossref] [PubMed]
- Komeda M, Fremes SE, David TE. Surgical repair of postinfarction ventricular septal defect. Circulation 1990;82:IV243-7. [PubMed]
- Isoda S, Imoto K, Uchida K, et al. Sandwich technique via right ventricle incision to repair postinfarction ventricular septal defect. J Card Surg 2004;19:149-50. [Crossref] [PubMed]
- Hosoba S, Asai T, Suzuki T, et al. Mid-term results for the use of the extended sandwich patch technique through right ventriculotomy for postinfarction ventricular septal defects. Eur J Cardiothorac Surg 2013;43:e116-20. [Crossref] [PubMed]
- Sugimoto T, Yoshii S, Yamamoto K, et al. A modified infarct exclusion technique: triple-patch technique for postinfarction ventricular septal perforation. J Thorac Cardiovasc Surg 2008;135:702-3. [Crossref] [PubMed]
- Okamoto Y, Yamamoto K, Asami F, et al. Early and midterm outcomes of triple patch technique for postinfarction ventricular septal defects. J Thorac Cardiovasc Surg 2016;151:1711-6. [Crossref] [PubMed]
- Tabuchi N, Tanaka H, Arai H, et al. Double-patch technique for postinfarction ventricular septal perforation. Ann Thorac Surg 2004;77:342-3. [Crossref] [PubMed]
- Kobayashi T, Mikamo A, Suzuki R, et al. Simple geometrical infarct exclusion technique with a single patch for postinfarction ventricular septal perforation. Ann Thorac Surg 2010;89:2049-52. [Crossref] [PubMed]
- Komeda M. Alternative method of patch implantation and creation for postinfarction ventricular septal defect repair by the infarct exclusion technique. J Thorac Cardiovasc Surg 2017;153:91-3. [Crossref] [PubMed]
- Cinq-Mars A, Voisine P, Dagenais F, et al. Risk factors of mortality after surgical correction of ventricular septal defect following myocardial infarction: Retrospective analysis and review of the literature. Int J Cardiol 2016;206:27-36. [Crossref] [PubMed]
- Barker TA, Ramnarine IR, Woo EB, et al. Repair of post-infarct ventricular septal defect with or without coronary artery bypass grafting in the northwest of England: a 5-year multi-institutional experience. Eur J Cardiothorac Surg 2003;24:940-6. [Crossref] [PubMed]
- Lundblad R, Abdelnoor M, Geiran OR, et al. Surgical repair of postinfarction ventricular septal rupture: risk factors of early and late death. J Thorac Cardiovasc Surg 2009;137:862-8. [Crossref] [PubMed]
- Sakaguchi G, Miyata H, Motomura N, et al. Surgical Repair of Post-Infarction Ventricular Septal Defect - Findings From a Japanese National Database. Circ J 2019;83:2229-35. [Crossref] [PubMed]
- Daubert MA, White JA, Al-Khalidi HR, et al. Cardiac remodeling after large ST-elevation myocardial infarction in the current therapeutic era. Am Heart J 2020;223:87-97. [Crossref] [PubMed]
- Pahuja M, Schrage B, Westermann D, et al. Hemodynamic Effects of Mechanical Circulatory Support Devices in Ventricular Septal Defect. Circ Heart Fail 2019;12:e005981. [Crossref] [PubMed]
- Patanè F, Centofanti P, Zingarelli E, et al. Potential role of the Impella Recover left ventricular assist device in the management of postinfarct ventricular septal defect. J Thorac Cardiovasc Surg 2009;137:1288-9. [Crossref] [PubMed]
- Ancona MB, Regazzoli D, Mangieri A, et al. Post-infarct ventricular septal rupture: early Impella implantation to delay surgery and reduce surgical risk. Cardiovasc Interv Ther 2017;32:381-5. [Crossref] [PubMed]
- Madjarov JM, Katz MG, Madzharov S, et al. Advantages of intraoperative implantation of Impella 5.5 SmartAssist in the Management of Acute Post-Infarction Ventricular Septal Defect with cardiogenic shock. J Cardiothorac Surg 2021;16:132. [Crossref] [PubMed]
- Møller-Helgestad OK, Poulsen CB, Christiansen EH, et al. Support with intra-aortic balloon pump vs. Impella2.5® and blood flow to the heart, brain and kidneys - an experimental porcine model of ischaemic heart failure. Int J Cardiol 2015;178:153-8. [Crossref] [PubMed]
- Via G, Buson S, Tavazzi G, et al. Early cardiac unloading with ImpellaCP™ in acute myocardial infarction with ventricular septal defect. ESC Heart Fail 2020;7:708-13. [Crossref] [PubMed]