Mitral valve repair in papillary muscle rupture
Introduction
Papillary muscle rupture (PMR) is a rare mechanical complication post-acute myocardial infarction (MI). It affects 0.07–0.26% of patients following MI and accounts for 5% of mortality after an infarct (1,2). It results in severe mitral valve regurgitation (MR), which is often accompanied by cardiogenic shock and pulmonary edema, requiring urgent management (3). The risk factors for PMR include older age, female sex, history of heart failure, delayed presentation following MI and chronic kidney disease. Medical management alone is associated with a mortality as high as 75% in the first 24 hours (4). PMR was first reported by Davison in 1948 (5) and the first mitral valve replacement for PMR was reported by Austen et al. in 1965 (6,7). There has not been a significant change in the management of patients presenting with acute PMR who require hemodynamic and ventilatory support followed by urgent or emergent mitral valve intervention.
Anatomy of the mitral sub-valvular apparatus
The mitral valve complex consists of the mitral valve annulus, anterior/posterior mitral leaflets, the sub-valvular apparatus [chordae tendineae and papillary muscles (PMs)] and the left ventricular wall (8,9). The PMs are located between the apex and the middle third of the left ventricle. They are connected by the chordae tendinea to the ventricular surface of the mitral valve leaflets. The sub-valvular apparatus as a whole function to prevent prolapse of the mitral valve into the left atrium during systole. Multiple attempts have been made to classify PMs, however, there is marked variation in their anatomy. The classical description is the presence of two PMs, however, in a post-mortem examination of PM this configuration of two PMs was only present in 3.4% of cases, whereas 43% had two groups of PMs, 31% had three groups and 21% had four groups (10). Other variations noted were PMs with ‘separate bases and fused apex’, ‘single base and divided apex’, ‘small projections’ of PMs, long PMs, ‘perforated’ PMs and those with a ‘base attached to a large bridge’ (10). These variations in PM may have implications in the feasibility and complexity of mitral valve repair (10).
PM dysfunction due to ischemia result in MR. In acute ischemic MR, the postero-medial PM is most commonly involved as it receives a single blood supply via the posterior descending artery whereas the antero-lateral PM receives dual supply from the left anterior descending and diagonal or the circumflex artery (2,9). Jouan et al. described four mechanisms of ischemic mitral valve prolapse (Figure 1): necrosis of a separate commissural head (inserted close to the annulus) with rupture of the anchorage of the commissural chord (Figure 1A); necrosis of a single head PM subdivided in multiple heads with partial rupture (Figure 1B); necrosis of a fenestrated PM with detachment of its main insertion: “incomplete” rupture (Figure 1C); single PM with complete and total rupture (Figure 1D) (11).
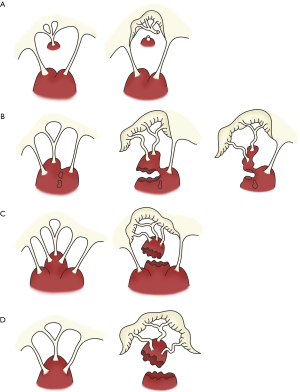
Clinical assessment
Patients presenting with breathlessness and or chest pain a week following a MI should be suspected to have a PMR. It is imperative a thorough history and clinical examination is carried out. On auscultation, patients who do have murmurs can have mid, late or holosystolic murmurs. Symptoms and signs will be in keeping with acute left heart failure with rapidly progressing cardiogenic shock and hypoxia (9).
Imaging
The gold standard investigation is echocardiography which typically reveals a mobile mass attached to a flailing mitral leaflet with evidence of severe eccentric MR. Left ventricular function may be normal or low-normal with regional wall abnormalities. Transthoracic echocardiography (TTE) has a sensitivity of 65–85% while transesophageal echocardiography (TEE) has a sensitivity of 92–100% (12). TEE is important in interrogating the mitral leaflets/sub-valvular apparatus to study the pathology/etiology of the regurgitant jet and various anatomical measurements that are required for the different treatment strategies. In most cases of PMR, the patients get massive MR due to bi-leaflet prolapse (muscle giving chords to multiple segments). As a result, on echocardiography you see severe MR or hyperdynamic ventricle without evidence of severe volume overload (very large left atrium or left ventricle), hence these patients go into acute pulmonary edema.
Pre-operative angiography is also important to determine the extent of coronary artery disease and may reveal occlusion of the infarct-related artery (7).
Mitral valve intervention
In the initial phase of presentation with acute MR, these patients require medical optimisation;
- Hemodynamic support with vasoactive drugs and mechanical circulatory support;
- Afterload reduction with intra-aortic balloon pump insertion (IABP);
- Acute pulmonary edema managed with intravenous diuretics and ventilatory support;
- Arrythmias treated using pharmacological or mechanical cardioversion;
- Percutaneous intervention of infarct-related artery may be required;
- Monitoring for other mechanical complications of MI.
Once the diagnosis of acute MR secondary to PMR has been made, definitive management requires correction of the MR without delay. The strategy for management of these patients due to ischemic PMR is dictated by the patient’s co-morbidities, the anatomy of the rupture, i.e., location and degree of the PM involvement (partial vs. complete), anatomy of coronary artery disease, the different intervention options available and local expertise guided by a consensus opinion from heart team discussions. Interventions to treat MR include percutaneous and surgical techniques. The practice to revascularize during concomitant mitral valve intervention in acute MR is variable.
The current gold standard treatment of PMR is surgery.
Mitral valve replacement
Chordal sparing mitral valve replacement is more commonly carried out in the setting of acute PMR. Multiple case series have been published with reported in-hospital mortality of 20–25% (13,14). Similarly, 5- and 10-year survival of 65–68% and 32–56%, respectively (15-17). Bouma et al. highlighted preservation of sub-valvular apparatus in mitral valve replacement is associated with better long-term survival (18).
On-pump mitral valve repair
Gillinov et al. in 2001 highlighted predictors of repair failure in chronic ischemic MR; presence of complex jet, lack of left internal mammary artery (LIMA) to left anterior descending artery (LAD) graft and lateral wall infarct (19). Mihos et al. proposed an echocardiographic-based treatment algorithm for repair over replacement (20). There have been multiple clinical trials and observational studies investigating outcomes after severe chronic ischemic MR, advocating mitral valve replacement over repair due to a higher incidence of recurrent MR in the latter group (21). There is scarcity of evidence of superiority of repair over replacement after PMR due to the rare nature of this entity.
In multiple observational studies, including case series and case reports of patients who developed PMR following an acute MI, the core principles of repair in these patients were largely based on Carpentier’s reconstructive valve techniques (11,13,14,18,22-37). The different surgical techniques used to repair the mitral valve in these studies included:
- Ruptured PM re-implantation to the adjacent PM: this involves re-implantation of the tip of the PM to adjacent healthy PM with a pledget re-enforced polytetrafluoroethylene (PTFE) suture. This also allows for height adjustment enabling good coaptation (27);
- Ruptured PM re-implantation at the base of the PM: the ruptured PM is implanted at the base of the healthy PM using pledgeted PTFE mattress sutures (31);
- Ruptured PM re-implantation in the left ventricle wall: this technique has been described by Carpentier, and involves lowering the ruptured PM to a sub-valvular level that restores the appropriate length of the chordae. A trench is created in the ventricular wall. Two mattress 4/0 sutures are passed through the cuff of the PM and through the trench as deep as possible which buries the cuff within the trench. Approximation sutures are placed to close the trench (Figure 2) (38);
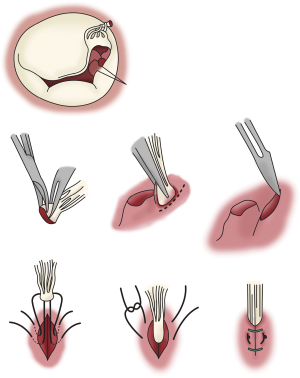 Figure 2 Implantation of ruptured papillary muscle into LV wall. A trench is created in the ventricular wall. Two mattress 4/0 sutures are passed through the cuff of the PM and through the trench as deep as possible which buries the cuff within the trench. Approximation sutures are placed to close the trench (24). LV, left ventricle; PM, papillary muscle.
Figure 2 Implantation of ruptured papillary muscle into LV wall. A trench is created in the ventricular wall. Two mattress 4/0 sutures are passed through the cuff of the PM and through the trench as deep as possible which buries the cuff within the trench. Approximation sutures are placed to close the trench (24). LV, left ventricle; PM, papillary muscle. - Use of Neochordae: pledgeted PTFE sutures are secured to the PM while the other ends are passed through the free edge of the leaflet. Once an adequate length of neochordae is achieved, allowing optimal coaptation, the sutures are then tied (38);
- Chordal translocation: this technique described by Carpentier involves transfer of secondary chordae from the ventricular aspect of the leaflet provided three conditions can be met: (I) chordae underneath the prolapse leaflet are selected; (II) the chordae are resilient; (III) annuloplasty ring is implanted to increase coaptation zone and reduced tension on the leaflet tissue and the transferred chordae (Figure 3) (38);
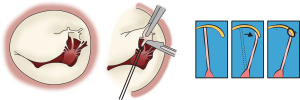 Figure 3 Chordal Translocation. Transfer of secondary chordae from the ventricular aspect of the leaflet to the leaflet edge (24).
Figure 3 Chordal Translocation. Transfer of secondary chordae from the ventricular aspect of the leaflet to the leaflet edge (24). - Resection of prolapse leaflet segment: these follow the same principles as described by Carpentier for degenerative valve disease (38);
- Annuloplasty ring or bands: in the majority of observational studies, patients undergoing mitral repair for PMR, annuloplasty rings or bands were used to support the mitral valve from future annular dilatation.
The preference for mitral valve repair when compared to mitral valve replacement appeared to be higher in earlier studies reducing to 17% in the most recent case series (13), reflecting surgeons’ preference for mitral valve replacement. Mitral valve repair failure rate ranged from 4% to 50% (11,13,14,18,22-37). This is likely to be due to a combination of edema, progression of tissue necrosis and lack of tensile strength in the ischemic tissues. Over the last three decades, apart from a reduction in the incidence of PMR to 0.07% from 1% to 5% as reported in the case series by Pahuja et al. (37), there has not been a significant improvement in surgical in-hospital mortality which has largely remained in the region of 25% (11,13,14,18,22-37). This is un-surprising as the conduct of the surgery and mechanical circulatory support has largely remained unchanged.
Off-pump transapical mitral valve repair
The transapical off-pump minimally invasive repair using the NeoChord DS1000 device (NeoChord, St Louis Park, MN, USA) is a novel technique for surgical repair of degenerative mitral valve disease (39). It allows for real time adjustment of the length of the neochords, providing optimal coaptation. Based on computed tomography reconstructions and intra-operative echocardiography, optimal incision level is determined usually at the fifth or sixth intercostal space. Successive dilatations of the left ventricular apical puncture site, the NeoChord DS1000 device (NeoChord, St Louis Park, MN, USA) is advanced over the guidewire into the left atrium and guided across the mitral valve. The posterior leaflet is captured and the neochords deployed and secured at the puncture site over Teflon strips (39).
In the case series described by Budra and colleagues, three patients developed PMR after presenting with inferior STEMI, resulting in severe MR and cardiogenic shock (40). All three patients underwent off pump neo-chord implantation ranging from two to three chords with a chord also used to secure the sub-valvular apparatus. All patients had moderate MR on discharge. They re-presented two to five months later with severe MR. The etiology of MR progression was due to rupture of native chords in two patients, entrapment of ruptured PM in the coaptation zone and rupture of artificial chordae in the other patient. Two patients underwent mitral valve replacement and one mitral valve repair with no in-hospital mortality. In the case series described by Heuts et al. seven patients underwent the above procedure for isolated P2 prolapse, a median of five chords were used and showed trivial MR at one-year follow-up (39). It might be that a higher number of chordae may be required to support the prolapsing leaflet in the setting of PMR, although it is a hypothetical observation. In a recent review of 203 patients following transapical off-pump NeoChord implantation, the incidence of moderate or higher residual MR at discharge was 13% (41).
The off-pump neo-chordae implantation in the case series by Budra and colleagues may serve as a bridge to recovery and subsequently to surgery in patients for whom surgery is precluded in the acute setting. Further studies will be required to determine the practicality of neo-chord implantation in PMR and their short- and long-term outcomes.
Other transapical devices include the Tendyne (Tendyne Holdings, LLC, Roseville, MN, USA) and the Intrepid (Medtronic, Minneapolis, MN, USA) which are self-expanding valves and are used in the setting of severe MR (42). However, to date there has not been any case descriptions of these valves being used in PMR. Future studies will be required to establish the feasibility of these valves in the setting of PMR.
Trans-catheter mitral valve repair
The current era of minimally invasive procedures is continuously evolving with multiple new medical innovations since the successful introduction of the trans-catheter aortic valve. However, the complex anatomy of the mitral valve, annulus, sub-valvular apparatus and proximity to the left ventricular outflow tract makes a single device that can address all mitral pathologies somewhat challenging.
The MitraClip device (Abbott Vascular, Abbott Park, IL, USA) is based on the principle of Alfieri’s stitch which is used to correct complex MR lesions (43). The MitraClip device (Abbott Vascular, Abbott Park, IL, USA) is a percutaneously implanted mechanical clip made of cobalt chromium arms covered by polyester fabric that grasp and coapts the mitral valve in a beating heart with the aid of echocardiography and fluoroscopy.
The current evidence for use of MitraClip is Class IIb in primary MR and Class IIa in secondary MR in patients who have prohibitive risks for surgery as per the 2021 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines for management of valvular heart disease (44).
MitraClips have also been used in acute ischemic PMR in patients with significant co-morbidities precluding them from mitral valve surgery (45-53). Between two to three clips were used in these patients creating a single orifice with mitral valve area of around 2.1 cm2,with a mean trans-mitral gradient ranging from 2 to 6 mmHg. All patients were successfully weaned from mechanical and inotropic support with trivial to moderate MR on discharge with no mortality (45-53).
These case reports (45-53) highlight novel use of MitraClip in PMR with successful outcomes in a patient population that were destined for extremely high-risk surgery or conservative management. It is pertinent to mention the slightly elevated trans-mitral gradients likely related to reduction in the mitral valve area. MitraClip-related mitral stenosis is associated with worse long-term outcomes and survival (54).
Further follow-up will be required to determine the long-term sequalae of MitraClip (Abbott Vascular, Abbott Park, IL, USA) although it appears a viable option in the short-term in addressing patients with cardiogenic shock due to PMR with no mortality in the high-risk patient cohort.
Revascularisation in PMR
In these critically ill patients, coronary angiography may be associated with ventricular arrythmias and hemodynamic compromise. Nevertheless, coronary angiography allows for identification of significant coronary artery disease that can be addressed during the same procedure and reduce the cross-clamp times allowing for a hybrid approach. Chevalier et al. showed a significant difference in survival in patients who underwent concomitant coronary artery bypass graft (CABG) along with mitral surgery. Similarly, a more recent case series by Massimi et al. also highlighted concomitant CABG being an independent predictor of survival (13,28). However, the benefit of concomitant revascularisation remains debatable.
Conclusions
In conclusion, in patients presenting with acute severe MR due to PMR, the optimal management strategy should be guided by dedicated mitral heart team discussions taking into consideration the clinical state of the patient, comorbidities, pathology of the valve and local technical expertise to ensure a successful patient outcome.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braunwald E, Maroko PR. Limitation of infarct size. Curr Probl Cardiol 1978;3:10-51. [Crossref] [PubMed]
- Damluji AA, van Diepen S, Katz JN, et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021;144:e16-35. [Crossref] [PubMed]
- Harari R, Bansal P, Yatskar L, et al. Papillary muscle rupture following acute myocardial infarction: Anatomic, echocardiographic, and surgical insights. Echocardiography 2017;34:1702-7. [Crossref] [PubMed]
- Nishimura RA, Schaff HV, Shub C, et al. Papillary muscle rupture complicating acute myocardial infarction: analysis of 17 patients. Am J Cardiol 1983;51:373-7. [Crossref] [PubMed]
- Davison S. Spontaneous rupture of a papillary muscle of the heart; a report of three cases and a review of the literature. J Mt Sinai Hosp N Y 1948;14:941-53. [PubMed]
- Austen WG, Sanders CA, Averill JH, et al. Ruptured papillary muscle. Report of a case with successful mitral valve replacement. Circulation 1965;32:597-601. [Crossref] [PubMed]
- Cohn LH. Surgical treatment of postinfarction rupture of a papillary muscle. Mayo Clin Proc 1992;67:1109-11. [Crossref] [PubMed]
- Krawczyk-Ożóg A, Hołda MK, Bolechała F, et al. Anatomy of the mitral subvalvular apparatus. J Thorac Cardiovasc Surg 2018;155:2002-10. [Crossref] [PubMed]
- Burton LV, Beier K. Papillary Muscle Rupture. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022 Jan.
- Gunnal SA, Wabale RN, Farooqui MS. Morphological variations of papillary muscles in the mitral valve complex in human cadaveric hearts. Singapore Med J 2013;54:44-8. [Crossref] [PubMed]
- Jouan J, Tapia M, C, Cook R, et al. Ischemic mitral valve prolapse: mechanisms and implications for valve repair. Eur J Cardiothorac Surg 2004;26:1112-7. [Crossref] [PubMed]
- Sochowski RA, Chan KL, Ascah KJ, et al. Comparison of accuracy of transesophageal versus transthoracic echocardiography for the detection of mitral valve prolapse with ruptured chordae tendineae (flail mitral leaflet). Am J Cardiol 1991;67:1251-5. [Crossref] [PubMed]
- Massimi G, Ronco D, De Bonis M, et al. Surgical treatment for post-infarction papillary muscle rupture: a multicentre study. Eur J Cardiothorac Surg 2022;61:469-76. [Crossref] [PubMed]
- Fujita T, Yamamoto H, Kobayashi J, et al. Mitral valve surgery for ischemic papillary muscle rupture: outcomes from the Japan cardiovascular surgery database. Gen Thorac Cardiovasc Surg 2020;68:1439-46. [Crossref] [PubMed]
- Tavakoli R, Weber A, Vogt P, et al. Surgical management of acute mitral valve regurgitation due to post-infarction papillary muscle rupture. J Heart Valve Dis 2002;11:20-5; discussion 26. [PubMed]
- Chen Q, Darlymple-Hay MJ, Alexiou C, et al. Mitral valve surgery for acute papillary muscle rupture following myocardial infarction. J Heart Valve Dis 2002;11:27-31. [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Koene BM, et al. Long-term survival after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg 2015;10:11. [Crossref] [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Klinkenberg TJ, et al. Mitral valve repair for post-myocardial infarction papillary muscle rupture. Eur J Cardiothorac Surg 2013;44:1063-9. [Crossref] [PubMed]
- Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2001;122:1125-41. [Crossref] [PubMed]
- Mihos CG, Santana O. Mitral valve repair for ischemic mitral regurgitation: lessons from the Cardiothoracic Surgical Trials Network randomized study. J Thorac Dis 2016;8:E94-9. [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Rankin JS, Feneley MP, Hickey MS, et al. A clinical comparison of mitral valve repair versus valve replacement in ischemic mitral regurgitation. J Thorac Cardiovasc Surg 1988;95:165-77. [Crossref] [PubMed]
- Deloche A, Jebara VA, Relland JY, et al. Valve repair with Carpentier techniques. The second decade. J Thorac Cardiovasc Surg 1990;99:990-1001; discussion 1001-2. [Crossref] [PubMed]
- David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1991;101:495-501. [Crossref] [PubMed]
- Kishon Y, Oh JK, Schaff HV, et al. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc 1992;67:1023-30. [Crossref] [PubMed]
- Yamanishi H, Izumoto H, Kitahara H, et al. Clinical experiences of surgical repair for mitral regurgitation secondary to papillary muscle rupture complicating acute myocardial infarction. Ann Thorac Cardiovasc Surg 1998;4:83-6. [PubMed]
- Fasol R, Lakew F, Wetter S. Mitral repair in patients with a ruptured papillary muscle. Am Heart J 2000;139:549-54. [Crossref] [PubMed]
- Chevalier P, Burri H, Fahrat F, et al. Perioperative outcome and long-term survival of surgery for acute post-infarction mitral regurgitation. Eur J Cardiothorac Surg 2004;26:330-5. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82. [Crossref] [PubMed]
- Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation 2008;118:1528-34. [Crossref] [PubMed]
- Park WK, Kim JB, Choo SJ. Repair of Acute Post Infarction Mitral Regurgitation with Papillary Muscle Reimplantation - A case report -. Korean J Thorac Cardiovasc Surg 2011;44:285-7. [Crossref] [PubMed]
- Schroeter T, Lehmann S, Misfeld M, et al. Clinical outcome after mitral valve surgery due to ischemic papillary muscle rupture. Ann Thorac Surg 2013;95:820-4. [Crossref] [PubMed]
- Vieira C, Gaspar A, Alvares Pereira M, et al. Ischemic rupture of the anterolateral papillary muscle. Rev Port Cardiol 2013;32:243-6. [Crossref] [PubMed]
- Lee SK, Heo W, Min HK, et al. A New Surgical Repair Technique for Ischemic Total Papillary Muscle Rupture. Ann Thorac Surg 2015;100:1891-3. [Crossref] [PubMed]
- Sultan I, Aranda-Michel E, Gleason TG, et al. Mitral valve surgery for acute papillary muscle rupture. J Card Surg 2018;33:484-8. [Crossref] [PubMed]
- Kilic A, Sultan I, Chu D, et al. Mitral Valve Surgery for Papillary Muscle Rupture: Outcomes in 1342 Patients From The Society of Thoracic Surgeons Database. Ann Thorac Surg 2020;110:1975-81. [Crossref] [PubMed]
- Pahuja M, Ranka S, Chauhan K, et al. Rupture of Papillary Muscle and Chordae Tendinae Complicating STEMI: A Call for Action. ASAIO J 2021;67:907-16. [PubMed]
- Carpentier AA, Adams D, Filsoufi F. Carpentier’s reconstructive valve surgery. 1st edition. Elsevier Health Sciences, 2010.
- Heuts S, Daemen JHT, Streukens SAF, et al. Preoperative Planning of Transapical Beating Heart Mitral Valve Repair for Safe Adaptation in Clinical Practice. Innovations (Phila) 2018;13:200-6. [Crossref] [PubMed]
- Budra M, Janušauskas V, Zorinas A, et al. Rescue transventricular off-pump mitral valve repair with artificial neochords for acute mitral regurgitation due to postinfarction papillary muscle rupture. JTCVS Tech 2021;10:231-42. [Crossref] [PubMed]
- Gerosa G, Nadali M, Longinotti L, et al. Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes. Ann Cardiothorac Surg 2021;10:131-40. [Crossref] [PubMed]
- Scott EJ, Rotar EP, Charles EJ, et al. Surgical versus transcatheter mitral valve replacement in functional mitral valve regurgitation. Ann Cardiothorac Surg 2021;10:75-84. [Crossref] [PubMed]
- Alfieri O, Denti P. Alfieri stitch and its impact on mitral clip. Eur J Cardiothorac Surg 2011;39:807-8. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Bilge M, Alemdar R, Ali S, et al. Percutaneous mitral valve repair with the MitraClip system in a patient with subacute severe mitral regurgitation caused by papillary muscle rupture. Anadolu Kardiyol Derg 2014;14:475-6. [Crossref] [PubMed]
- Wolff R, Cohen G, Peterson C, et al. MitraClip for papillary muscle rupture in patient with cardiogenic shock. Can J Cardiol 2014;30:1461.e13-4. [Crossref] [PubMed]
- Bahlmann E, Frerker C, Kreidel F, et al. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg 2015;99:e41-2. [Crossref] [PubMed]
- Valle JA, Miyasaka RL, Carroll JD. Acute Mitral Regurgitation Secondary to Papillary Muscle Tear: Is Transcatheter Edge-to-Edge Mitral Valve Repair a New Paradigm? Circ Cardiovasc Interv 2017;10:e005050. [Crossref] [PubMed]
- Yasin M, Nanjundappa A, Annie FH, et al. Use of MitraClip for Postmyocardial Infarction Mitral Regurgitation Secondary to Papillary Muscle Dysfunction. Cureus 2018;10:e3065. [Crossref] [PubMed]
- Villablanca PA, Wang DD, Lynch D, et al. Successful MitraClip XTR for Torrential Mitral Regurgitation Secondary to Papillary Muscle Rupture as a Complication of Acute Myocardial Infarction. Structural Heart 2019;3:352-5. [Crossref]
- Papadopoulos K, Chrissoheris M, Nikolaou I, et al. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep 2019;3:ytz001. [Crossref] [PubMed]
- Cannata F, Sanz-Sánchez J, Cozzi O, et al. Percutaneous mitral valve repair in acute mitral regurgitation. G Ital Cardiol (Rome) 2021;22:32S-38S. [PubMed]
- Hayek A, Grinberg D, Derimay F, et al. Transcatheter Mitral Valve Repair to Treat Postmyocardial Infarction Papillary Muscle Rupture. J Cardiovasc Echogr 2021;31:104-6. [PubMed]
- Schnitzler K, Hell M, Geyer M, et al. Complications Following MitraClip Implantation. Curr Cardiol Rep 2021;23:131. [Crossref] [PubMed]








