Left ventricular pseudoaneurysm: the niche of post-infarction mechanical complications
Introduction
Left ventricular pseudoaneurysm (LVP) represents a very rare but potentially lethal mechanical complication of acute myocardial infarction (AMI), representing a peculiar subtype of left ventricular (LV) free-wall rupture (FWR). Indeed, although FWR may present as either a blowout or an oozing rupture, characterized by either massive, acute or subacute blood tearing from the heart wall into the pericardial sac, LVP cardiac rupture is generally contained by adherent pericardium or scar tissue, thereby involving only a portion of the wall thickness.
While the incidence of post-AMI complications significantly decreased after the development and wide adoption of early percutaneous revascularization strategies, robust data on the real incidence of LVP is still lacking, even though it has been traditionally described to occur in about 0.2–0.5% of AMI cases (1-4).
Patient characteristics and comorbidities associated with a higher risk of developing LVP include older age, male sex, hypertension, as well as inferior and lateral AMI (5).
LVPs are characterized by a small, narrow neck communication developing from a tear within the ventricular wall resulting from AMI injury, which connects with a larger aneurysmal sac containing blood and thrombi. This cavity is lined with fibrous pericardial tissue devoid of myocardial cells. It is distinguished from a LV true aneurysm that develops in ischemic cardiomyopathy, as the latter is the result of post-AMI adverse remodeling occurring from the necrotic scar and resulting in wall thinning and progressive ventricular dilatation in accordance with Laplace’s law (6-9).
Classification
According to the time from AMI onset, LVPs may be classified into acute (within 2 weeks), subacute (from 2 weeks to 3 months) and chronic (beyond 3 months). Such groups are also generally characterized by a different clinical presentation (10,11).
Localization
LVPs occur more frequently after inferolateral/posterior AMI, and this localization is quite peculiar of the disease, compared to true aneurysms which often develop from anterior AMI (5,9). Posterior pseudoaneurysms have been identified in 48% of the cases, compared to the less frequent lateral (28%) and apical (20%) ones (5,12). The reason for a lower incidence of anterior LVP might be attributed to the fact that anterior LV ruptures can be seldom contained by the adjacent pericardium and therefore tend to develop more often into a complete FWR. As a matter of fact, and from the diagnostic point of view, it has been observed that a posterior localization itself might be considered suggestive of LVP rather than of true aneurysm.
Concomitantly, it is also well known that an extensive AMI of the posterior LV wall can also involve the posteromedial papillary muscle, thereby resulting in mitral regurgitation (MR), which may be due either to papillary muscle rupture or dysfunction causing acute organic or functional MR, respectively.
Clinical presentation
The clinical presentation is highly variable and nonspecific. Indeed, LVPs are often detected incidentally during the diagnostic workup for other presenting conditions (i.e., angina, pulmonary edema or decompensated heart failure). The most common symptoms include dyspnea, chest pain, congestive heart failure, dizziness or altered mental status, yet more than 10% of patients are asymptomatic (5,9,13,14). Clinical manifestations resulting from systemic embolization may also be observed in patients with voluminous thrombi, usually present in large pseudoaneurysms (>3 cm) (15).
As the pseudoaneurysm cavity enlarges, other structural conditions may occur, such as MR resulting from excessive tethering, which may worsen the dyspnea. Moreover, pseudoaneurysm expansion can lead to an ab extrinseco compression of the coronary arteries during systole, thereby causing angina at rest (16,17).
On physical examination, muffled heart sounds, pericardial rub and especially a holosystolic or “to-and-fro” murmur closely resembling that of MR are among the most common signs (5,11,12,16).
Timing of occurrence
Although large studies are not available on this severe condition, many authors reported great variability in the timing of LVP presentation, ranging from a few days (less than a week) to several years after AMI. However, most often LVP is diagnosed within 60 days from the ischemic event (5,14,18,19).
Diagnostic workup
Diagnostic workup of LVP parallels the investigations generally performed for the assessment of coronary artery disease. Electrocardiography usually shows nonspecific ST-segment alterations in the majority of patients, though ST-elevation may be identified in about 20% of subjects (20,21). An enlarged cardiac silhouette is the most common finding on chest X-ray.
Angiography
Ventricular angiography has been traditionally considered the ‘gold standard’ for the diagnosis of pseudoaneurysms. Angiographic distinctive features of LVP include delayed release of contrast into the noncontracting cavity and the lack of surrounding coronary arteries (Figure 1) (22,23).
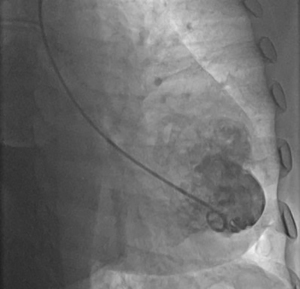
However, this diagnostic technique is not performed in all patients, as it can be time-consuming and carries a risk of pseudoaneurysm rupture due to LV catheter entrapment or dye injection. We do not recommend this test for patients with large thrombi due to the high risk of embolization, and for patients with large pseudoaneurysms because a mild increase in the intracavitary pressure or a small lesion caused by the tip of the catheter on the ventricular wall may have fatal consequences.
On the other hand, if the patient’s clinical condition is stable, coronary angiography should be routinely performed, in order to precisely assess the underlying coronary disease and to plan any needed revascularization, either percutaneous or surgical.
Echocardiography
Transthoracic echocardiography (TTE) is the first-line imaging modality to identify LVP even in asymptomatic patients. TTE is also particularly helpful for the differential diagnosis of this condition and many authors have highlighted how this diagnostic tool may be more accurate than ventricular angiography, even if sometimes non-conventional views are required to identify small wall lesions (18).
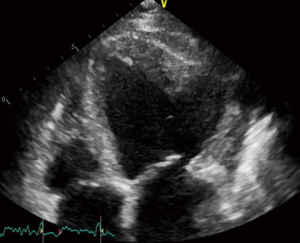
On TTE, LVPs present the following pathognomonic features: a narrow neck at the site of the rupture with an abrupt transition from normal myocardium to aneurysmatic scar tissue, with the diameter of the neck being 50% smaller than the maximum diameter of the aneurysmal cavity (Figure 2). With the aid of the color Doppler, pseudoaneurysm can be confirmed as an unusual continuous flow signal extending from the LV into the aneurysmal cavity through the neck (12,24). Among the advantages provided by TTE, it allows a more accurate characterization of the anatomy, size and localization of the rupture, and the identification and quantification of possible associated valvular dysfunctions. Moreover, it may help assess for the presence of intracavitary thrombi. Adding 3D-echocardiography views may also provide further anatomic information on the rupture (25).
Computed tomography (CT) and cardiac magnetic resonance (CMR)
CT and CMR are the preferred modalities for distinguishing pseudoaneurysm from true aneurysm, as they provide better spatial resolution and tissue definition to differentiate myocardium from scar tissue and from adjacent pericardium (21).
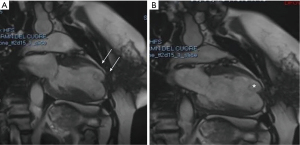
Cardiac CT allows non-invasive, rapid and detailed visualization of the aneurysmal cavity and of the ventricular rupture site. In patients presenting with cardiac symptoms and with labile hemodynamic stability, CT may be also useful in the differential diagnosis of other surgical emergent conditions, such as acute aortic syndromes (26). The adoption of CMR to diagnose LVP was firstly reported in 1991 (27). A typical feature that characterized LVP with respect to true aneurysm is the presence of a markedly delayed enhancement of the surrounding pericardium. The peculiar accuracy of CMR in tissue characterization also helps distinguish between pericardium, thrombus and myocardial tissue (Figure 3). Moreover, given its spatial resolution, detailed information about the size and location of the rupture may also be acquired (12,28,29).
Prognosis
The natural history of LVP is not well established, even though it is well accepted that this severe condition has a poor prognosis because of the high risk of rupture (30–45%). It should be highlighted that the risk of LVP rupture is inversely correlated with timing from AMI onset. Moreover, LVP occurring in the first days after AMI are those at highest risk of rupture. The risk of rupture is lower for chronic pseudoaneurysms (5,30,31).
Urgent surgical intervention for acute LVP has been advocated already in 1975 by Gueron and colleagues. According to many other authors later on, the diagnosis of pseudoaneurysm should always be an indicator for urgent surgical intervention, if occurring within 3 months from AMI, or unless surgical risk is prohibitive (5,32,33).
However, for an asymptomatic, small (<3 cm) and stable pseudoaneurysm, conservative medical management may be an option, with strict clinical and echocardiographic monitoring (7). Conversely, for larger pseudoaneurysms, conservative management is associated with a high mortality at 2 years (50%). Therefore, unless there are clinical contraindications, in these patients surgical treatment should be promptly performed (34).
Surgical treatment
Although LVP has been described for the first time by Corvisart in 1797, only in 1957 did Smith et al. report the first successful surgical repair (35).
Since then, surgery is the first-line treatment for LVP. Although no dedicated guidelines exist for this post-AMI complication, surgery has been associated with a lower in-hospital mortality compared to conservative treatment (23% vs. 48%). Recent advances in surgical techniques have appeared to further decrease the perioperative mortality to less than 10% (5,33,34,36).
Unlike acute and subacute LVPs, indications for the treatment of chronic pseudoaneurysm is still controversial, because the risk of rupture and embolism should be weighed against the estimated risk of surgery, and the decision is mainly guided by the clinical presentation and the severity of symptoms. Indeed, 10–20% of chronic pseudoaneurysms are diagnosed incidentally, and Moreno et al. reported a cumulative survival of 74.1% at 4 years for chronic pseudoaneurysms treated medically (31). Any increase in size, however, should suggest the need for prompt intervention.
Therefore, as reported by different authors, it seems reasonable to suggest surgery for patients presenting with congestive heart failure, or in cases where the pseudoaneurysm has a diameter greater than 3 cm, or if there is evidence of aneurysm expansion or rupture (7,9,11,15,19,34).
Surgical approach (median sternotomy vs. thoracotomy) depends on the patient’s surgical status (first surgery vs. reintervention), rupture localization (anterior vs. postero-basal), timing of rupture (acute vs. chronic), need of concomitant procedure and hemodynamic stability.
For chronic LVPs, the neck can be closed directly, taking advantage of the stronger fibrous scar edges.
In acute LVPs, closure of the freshly necrotic myocardium may be achieved by suturing a synthetic (e.g., Dacron or Teflon) or pericardial patch. When the rupture is larger or located near the base of the heart, a patch repair may be safer in order to avoid excessive traction on friable myocardium that is at higher risk of suture dehiscence. Concomitant procedures are also advocated when needed. Particular interest should be devoted to coronary artery bypass grafting, as the effectiveness and impact of coronary revascularization on survival still represent a matter of debate in the setting of post-AMI mechanical complications, especially concerning the infarct-related artery supplying the wall region involved in the rupture (15).
LVPs of the anterior wall or near the body of the sternum may be particularly dangerous for the patient who needs to undergo surgery. Therefore, in some situations it could be safer to be prepared to institute cardiopulmonary bypass (CPB) rapidly via the femoral vessels in case of sudden rupture at time of sternotomy (37). Moreover, for patients presenting in an extremely severe condition and possibly even cardiogenic shock, mechanical circulatory support devices, such as intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation (ECMO) can be useful tools to support the patient and achieve hemodynamic stability while waiting for surgery (38).
LVP repair may be accomplished from outside or inside the heart. The advantages of internal repair include better exposure of the sub-valvular apparatus, making the repair straightforward; possibility to repair additional cardiac abnormalities simultaneously; and correct visualization of the left circumflex coronary artery which is better protected than with external repair. Antunes et al. described a trans-atrial approach through a left atriotomy parallel to the posterior part of the annulus that exposes the aneurysmal cavity and neck (39). The advantages of external repair, on the other hand, include easier access to the pseudoaneurysm that is not limited by the mitral annulus and is not influenced by the presence of a previously positioned prosthesis; and possibility to completely resect the pseudoaneurysm (11,40).
The ideal surgical techniques to be adopted for LVP repair should be carefully chosen according to several characteristics, including rupture localization, and generally mirror the techniques described traditionally to correct LV true aneurysm. Since Dor’s procedure, several variations have been developed and techniques have been further refined.
For example, in the case of an anterior-apical pseudoaneurysm, the Dor technique may be adopted, because it allows reduction of the pseudoaneurysm neck by means of purse-string sutures, while with a Dacron patch, it allows closure of the defect by restoring the proper conical LV shape without excessively reducing ventricular volume (41,42).
In the case of an inferior pseudoaneurysm, the more convenient approach is through median sternotomy, as lateral thoracotomy appears unfavorable to reach the rupture site. When LVP involves the posterior LV wall, the simple patch repair is usually suggested in order to avoid distortion within proximity of the mitral valve apparatus (43).
When closing the LV cavity, Teflon felt strips should always be put between the sutures and the ventricular wall to protect myocardial tissue from potentially traumatic positioning of the suture and to avoid bleeding from the suture line or needle holes.
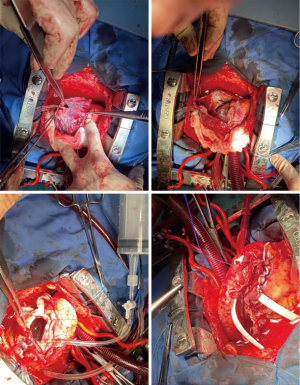
At our institution, pseudoaneurysm repair is performed under cardioplegic arrest after central CPB institution. The ventricular wall is thoroughly examined to identify the correct location of the pseudoaneurysm and the condition of the involved tissue. Then, the cavity is opened in the middle of the scar starting from the mid portion and proceeding towards the apex. If present, thrombi are removed. A reference stitch is placed at the apical portion of the ventricle as a landmark for geometrical ventricular reconstruction. A mattress suture reinforced with two strips of Teflon felt is performed along the neck of the pseudoaneurysm in order to restore the appropriate ventricular cavity. Since July 2001, if necessary, a pre-shaped device is used to restore left ventricle geometry. This device is deflated and removed before ending the suture. The remaining tissue is oversewn with a running suture to provide more resistance. A further mattress suture reinforced with two strips of Teflon felt is then ultimately performed to reinforce the suture lines and for hemostatic purposes (Figure 4).
A triple-layer patch repair has been described in 2016 by Elgharably et al. as the “Empanada Patch”. In this case, the authors prepared a triple-layer patch by folding bovine pericardium over a Dacron patch. Such a technique has the advantage of providing further rigidity to the patch, therefore making it less prone to bulging out during systole (44).
In-hospital mortality for the surgical treatment of LVP remains invariably high, according to various reports, and ranges from 13% to 28%. Interestingly, Komeda et al. (15) observed that such a high mortality is not associated with the technical difficulties related to pseudoaneurysm repair, but rather to the poor LV function often affecting these patients and to the possible need for concomitant mitral valve surgery. Nevertheless, most authors agree that such relatively high in-hospital mortality is acceptable, considering the high risk of death associated with pseudoaneurysm rupture. Even though recent improvements in surgical techniques and patient management have led to higher survival rates after LVP repair, a higher risk of death has been found in older patients presenting with advanced heart failure and requiring concomitant procedures on the mitral valve (5,11,15,34).
Although rare, the recurrence of LVP following surgical repair is not an impossible event and it has been reported to occur in about 5% of cases (5).
Finally, for patients presenting in an acceptable general condition, but in whom LVP repair is not technically feasible, the possibility of heart transplantation has also been reported (45).
Trans-catheter closure
For patients at extremely high surgical risk or deemed inoperable, the percutaneous closure of LVP has been described as a feasible alternative to surgery.
The first trans-catheter treatment for a patient with cardiac rupture has been described by Joho et al. in 2002, who successfully performed an intrapericardial fibrin-glue injection to repair an oozing-type FWR in an 82-year-old patient who presented with cardiac tamponade (46).
In 2004, Clift et al. performed the first percutaneous device closure of a pseudoaneurysm with an Ampltazer occluder in a patient who had been previously operated for surgical ventricular reconstruction and coronary artery bypass grafting (47).
Since then, several case reports described the successful adoption of a variety of closure devices, including Amplatzer septal occluder and ventricular septal defect occluder, and coils. Both septal occluders and coils present peculiar characteristics that may guide the choice for trans-catheter pseudoaneurysm closure. Although coils are technically easier to be deployed and can be used for any ventricular location with small concerns related to their mechanical effects on the LV wall, especially in the setting of small and medium pseudoaneurysms, they may result in incomplete closure. On the other hand, septal occluders are technically more demanding but may help accomplish complete closure even for larger pseudoaneurysms. However, their mechanical effects must be carefully assessed, as they may lead to valve dysfunction and coronary obstruction. For this reason, it is also not advisable to choose septal occluder for basal pseudoaneurysms (48). Some authors, however, also reported the percutaneous closure of LVP with a combined adoption of both coils and Amplatzer occluder (49-51).
However, given the technical complexity of such approach, a complete pre-operative evaluation, including all the anatomical characteristics (size, morphology, localization, length and depth of the neck and analysis of nearby anatomical structures) is mandatory in order to establish the appropriate device selection and access route. For instance, the neck length might guide the decision of the appropriate closure device (e.g., duct occluder or vascular plug in long necks, septal occluder in short and larger necks).
Access routes for percutaneous LVP closure include retrograde transaortic approach, transapical, anterograde trans-septal and direct thoracotomy approach.
Percutaneous closure of LVP has some technical limitations, mainly due to the need for robust and defined borders for secure anchoring of the device. The presence of a well-defined neck is essential to give a safe and stable device position. For patients with a large and poorly defined landing zone at the level of the neck, trans-catheter closure may not be feasible, due to the high risk of device dislodgement and embolization.
Nevertheless, no large studies are available to guide the optimal device selection for these patients and a multidisciplinary discussion is always advisable to identify the most appropriate and patient-tailored treatment.
Conclusions
LVP is a rare, but severe post-infarction mechanical complication, associated with potentially catastrophic consequences. Although not always occurring early after AMI, a high clinical suspicion should aid in the rapid and correct diagnosis as soon as symptoms develop, in order to immediately proceed with the more appropriate treatment (generally surgical) and to prevent the potentially lethal complications associated with LVP progression, namely rupture. Correct preoperative evaluation includes anatomical characterization in order to plan the most appropriate surgical approach for the type of lesion. Suboptimal in-hospital survival despite prompt surgery might be attributable to the generally poor LV function of patients presenting in such a setting. Finally trans-catheter closure might represent a potentially beneficial alternative to surgery for a subgroup of patients deemed at excessive risk for traditional intervention.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: RL is a consultant for Medtronic, Getinge and LivaNova, and Member of the advisory board of Eurosets and Fresenius/Xenios. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Figueras J, Alcalde O, Barrabés JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008;118:2783-9. [Crossref] [PubMed]
- Csapo K, Voith L, Szuk T, et al. Postinfarction left ventricular pseudoaneurysm. Clin Cardiol 1997;20:898-903. [Crossref] [PubMed]
- Erdim R, Yildirimturk O, Polat B, et al. Left ventricular pseudoaneurysm complicating inferior myocardial infarction: a case report. Int J Angiol 2011;20:107-10. [Crossref] [PubMed]
- Catherwood E, Mintz GS, Kotler MN, et al. Two-dimensional echocardiographic recognition of left ventricular pseudoaneurysm. Circulation 1980;62:294-303. [Crossref] [PubMed]
- Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol 1998;32:557-61. [Crossref] [PubMed]
- Davies MJ. Ischaemic ventricular aneurysms: true or false? Br Heart J 1988;60:95-7. [Crossref] [PubMed]
- Eren E, Bozbuga N, Toker ME, et al. Surgical treatment of post-infarction left ventricular pseudoaneurysm: a two-decade experience. Tex Heart Inst J 2007;34:47-51. [PubMed]
- Yaymaci B, Bozbuga N, Balkanay M. Unruptured left ventricular pseudoaneurysm. Int J Cardiol 2001;77:99-101. [Crossref] [PubMed]
- Yeo TC, Malouf JF, Oh JK, et al. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med 1998;128:299-305. [Crossref] [PubMed]
- Gan HL, Zhang JQ. Diagnosis and surgical treatment of post-infarction left ventricular pseudoaneurysm. Chin Med J (Engl) 2009;122:232-5. [PubMed]
- Prêtre R, Linka A, Jenni R, et al. Surgical treatment of acquired left ventricular pseudoaneurysms. Ann Thorac Surg 2000;70:553-7. [Crossref] [PubMed]
- Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm. A review of the literature. Chest 1997;111:1403-9. [Crossref] [PubMed]
- Contuzzi R, Gatto L, Patti G, et al. Giant left ventricular pseudoaneurysm complicating an acute myocardial infarction in patient with previous cardiac surgery: a case report. J Cardiovasc Med (Hagerstown) 2009;10:81-4. [Crossref] [PubMed]
- Yeo TC, Malouf JF, Reeder GS, et al. Clinical characteristics and outcome in postinfarction pseudoaneurysm. Am J Cardiol 1999;84:592-5, A8.
- Komeda M, David TE. Surgical treatment of postinfarction false aneurysm of the left ventricle. J Thorac Cardiovasc Surg 1993;106:1189-91. [Crossref] [PubMed]
- Kansiz E, Hatemi AC, Tongut A, et al. Surgical treatment of a giant postero-inferior left ventricular pseudoaneurysm causing severe mitral insufficiency and congestive heart failure. Ann Thorac Cardiovasc Surg 2012;18:151-5. [Crossref] [PubMed]
- Ozdogru I, Arinc H, Dogan A, et al. Left ventricular pseudoaneurysm causing coronary angiography images resembling myocardial bridging. Int J Cardiovasc Imaging 2007;23:135-8. [Crossref] [PubMed]
- Atik FA, Navia JL, Vega PR, et al. Surgical treatment of postinfarction left ventricular pseudoaneurysm. Ann Thorac Surg 2007;83:526-31. [Crossref] [PubMed]
- Fedakar A, Bugra O, Onk A, et al. Repair of left ventricular pseudoaneurysms. Asian Cardiovasc Thorac Ann 2010;18:39-43. [Crossref] [PubMed]
- March KL, Sawada SG, Tarver RD, et al. Current concepts of left ventricular pseudoaneurysm: pathophysiology, therapy, and diagnostic imaging methods. Clin Cardiol 1989;12:531-40. [Crossref] [PubMed]
- Bisoyi S, Dash AK, Nayak D, et al. Left ventricular pseudoaneurysm versus aneurysm a diagnosis dilemma. Ann Card Anaesth 2016;19:169-72. [Crossref] [PubMed]
- Bekkers SC, Borghans RA, Cheriex EC. Ventricular pseudoaneurysm after subacute myocardial infarction. Int J Cardiovasc Imaging 2006;22:791-5. [Crossref] [PubMed]
- al-Saadon K, Walley VM, Green M, et al. Angiographic diagnosis of true and false LV aneurysms after inferior wall myocardial infarction. Cathet Cardiovasc Diagn 1995;35:266-9. [Crossref] [PubMed]
- Sutherland GR, Smyllie JH, Roelandt JR. Advantages of colour flow imaging in the diagnosis of left ventricular pseudoaneurysm. Br Heart J 1989;61:59-64. [Crossref] [PubMed]
- Little SH, Ramasubbu K, Zoghbi WA. Real-time 3-dimensional echocardiography demonstrates size and extent of acute left ventricular free wall rupture. J Am Soc Echocardiogr 2007;20:538.e1-3. [Crossref] [PubMed]
- Ghersin E, Kerner A, Gruberg L, et al. Left ventricular pseudoaneurysm or diverticulum: differential diagnosis and dynamic evaluation by catheter left ventriculography and ECG-gated multidetector CT. Br J Radiol 2007;80:e209-11. [Crossref] [PubMed]
- Harrity P, Patel A, Bianco J, et al. Improved diagnosis and characterization of postinfarction left ventricular pseudoaneurysm by cardiac magnetic resonance imaging. Clin Cardiol 1991;14:603-6. [Crossref] [PubMed]
- Konen E, Merchant N, Gutierrez C, et al. True versus false left ventricular aneurysm: differentiation with MR imaging--initial experience. Radiology 2005;236:65-70. [Crossref] [PubMed]
- Sørensen MB, Moat NE, Mohiaddin RH. Images in cardiovascular medicine. False left ventricular aneurysm documented by magnetic resonance imaging. Circulation 2002;105:1734. [Crossref] [PubMed]
- Webb J, Gemmell RM, Al-Fakih K, et al. Medical treatment of left ventricular pseudoaneurysms. QJM 2016;109:213-4. [Crossref] [PubMed]
- Moreno R, Gordillo E, Zamorano J, et al. Long term outcome of patients with postinfarction left ventricular pseudoaneurysm. Heart 2003;89:1144-6. [Crossref] [PubMed]
- Gueron M, Wanderman KL, Hirsch M, et al. Pseudoaneurysm of the left ventricle after myocardial infarction: A curable form of myocardial rupture. J Thorac Cardiovasc Surg 1975;69:736-42. [Crossref] [PubMed]
- Al Saidi K, Malik SA, Albulushi A, et al. Left Ventricular Pseudoaneurysm Complicated With Very Late Rupture 5 Years After Myocardial Infarction. JACC Case Rep 2019;1:569-72. [Crossref] [PubMed]
- Narin C, Ege E, Ozkara A, et al. Surgical treatment of postinfarction pseudoaneurysms of the left ventricle. J Card Surg 2008;23:294-8. [Crossref] [PubMed]
- Smith RC, Goldberg H, Bailey CP. Pseudoaneurysm of the left ventricle: diagnosis by direct cardioangiography; report of two cases successfully repaired. Surgery 1957;42:496-510. [PubMed]
- Prifti E, Bonacchi M, Baboci A, et al. Surgical treatment of post-infarction left ventricular pseudoaneurysm: Case series highlighting various surgical strategies. Ann Med Surg (Lond) 2017;16:44-51. [Crossref] [PubMed]
- Bauer M, Musci M, Pasic M, et al. Surgical treatment of a chest-wall penetrating left ventricular pseudoaneurysm. Ann Thorac Surg 2000;70:275-6. [Crossref] [PubMed]
- Holtackers RJ, Ter Bekke RMA, Bijvoet GP, et al. A Boolean Dilemma: True or False Aneurysm? JACC Case Rep 2021;3:112-6. [Crossref] [PubMed]
- Antunes MJ. Submitral left ventricular aneurysms. Correction by a new transatrial approach. J Thorac Cardiovasc Surg 1987;94:241-5. [Crossref] [PubMed]
- Ono M, Wolf RK. Left ventricular pseudoaneurysm late after mitral valve replacement. Ann Thorac Surg 2002;73:1303-5. [Crossref] [PubMed]
- Menicanti L, Di Donato M. The Dor procedure: what has changed after fifteen years of clinical practice? J Thorac Cardiovasc Surg 2002;124:886-90. [Crossref] [PubMed]
- Dor V, Di Donato M, Sabatier M, et al. Left ventricular reconstruction by endoventricular circular patch plasty repair: a 17-year experience. Semin Thorac Cardiovasc Surg 2001;13:435-47. [Crossref] [PubMed]
- O'Flynn E, Purkayastha S, Athanasiou T, et al. Repair of a giant left ventricular pseudoaneurysm in a Jehovah's Witness. Asian Cardiovasc Thorac Ann 2006;14:328-30. [Crossref] [PubMed]
- Elgharably H, Halbreiner MS, Shoenhagen P, et al. Repair of left ventricular pseudoaneurysm with the triple patch technique (Empanada Patch). Interact Cardiovasc Thorac Surg 2016;22:116-7. [Crossref] [PubMed]
- Jáuregui B, Sobrino JM, Lage E, et al. Giant unruptured left ventricular pseudoaneurysm as a rare cause of heart failure after an unnoticed coronary ischaemic event. Eur Heart J Cardiovasc Imaging 2013;14:720. [Crossref] [PubMed]
- Joho S, Asanoi H, Sakabe M, et al. Long-term usefulness of percutaneous intrapericardial fibrin-glue fixation therapy for oozing type of left ventricular free wall rupture: a case report. Circ J 2002;66:705-6. [Crossref] [PubMed]
- Clift P, Thorne S, de Giovanni J. Percutaneous device closure of a pseudoaneurysm of the left ventricular wall. Heart 2004;90:e62. [Crossref] [PubMed]
- Kumar PV, Alli O, Bjarnason H, et al. Percutaneous therapeutic approaches to closure of cardiac pseudoaneurysms. Catheter Cardiovasc Interv 2012;80:687-99. [Crossref] [PubMed]
- Lin CH, Balzer D, Lasala J. Transcatheter closures of a postinfarction ventricular septal defect and late ventricular pseudoaneurysm. J Invasive Cardiol 2010;22:E132-7. [PubMed]
- Acharya D, Nagaraj H, Misra VK. Transcatheter closure of left ventricular pseudoaneurysm. J Invasive Cardiol 2012;24:E111-4. [PubMed]
- Yudi MB, Love B, Nadir A, et al. Percutaneous Closure of Left Ventricular Pseudoaneursym With Septal Occluder Device and Coils: A Multimodality Imaging Approach. JACC Cardiovasc Interv 2017;10:e159-61. [Crossref] [PubMed]






