Systematic review and meta-analysis of the mechanical complications of ischemic heart disease: papillary muscle rupture, left ventricle rupture and post-infarct ventricular septal defect
Introduction
Improvements in revascularisation, including pharmacological, catheter-based and surgical, have resulted in improved outcomes for patients with acute myocardial infarction (AMI), leading to decreased frequency of mechanical complications (1,2). Large infarcts, delayed hospital presentation and a lack of tissue perfusion due to poor coronary flow post-intervention, are risk factors for mechanical complications including; acute mitral regurgitation (MR) secondary to papillary muscle rupture (PMR), ventricular septal defect (VSD), pseudoaneurysm and free wall rupture (FWR) (3). Although the incidence of mechanical complications following AMI has declined at <1%, the mortality rates have not decreased (4). Patients who go on to develop mechanical complications tend to be older, female, have a history of cardiac failure, chronic kidney disease and are often presenting with their first AMI (3).
Papillary muscle rupture presents 3–5 days following transmural inferior or lateral infarct, with resultant posteromedial PMR, typically in acute pulmonary oedema and cardiogenic shock. The incidence of acute severe MR from PMR is around 0.05–0.26%, with a reported mortality rate of 10–40% (3). Ventricular septal defects carry a high mortality of 30–40% acutely and up to 80% at 30 days, typically presenting 3–5 days after transmural infarct, with symptoms ranging from isolated murmur to circulatory collapse with cardiogenic shock (3). Free wall rupture remains the most reported mechanical complication, however, the true incidence is unknown as it usually presents as an out-of-hospital sudden cardiac death within 7 days, following a transmural infarct (4). Left ventricular pseudoaneurysms typically present weeks to years following infarct, when cardiac rupture is contained by pericardial adhesions and may be asymptomatic or present with chronic heart failure, chest pain or dyspnoea (4). Given their high risk for progression and rupture, consideration of urgent surgical repair is prudent with an estimated mortality of <10% (3).
All mechanical complications of ischaemic heart disease (IHD) conventionally require surgical intervention. However, with improvements in techniques and technology, select patients can be managed with a purely percutaneous, transcatheter strategy. Most mechanical complications are high-acuity and time-sensitive surgical emergencies, requiring prompt discussion with cardiac surgery, cardiac intensivists and cardiologists to diagnose and manage. Given the vast array of management options with advances in transcatheter technology, multidisciplinary discussions involving the patient and family’s preference for care should be undertaken in this population with a high mortality risk, regardless of treatment strategy (4).
Aim
Through systematic review, this study aims to synthesise the collective experience of percutaneous treatment of the mechanical complications of ischaemic heart disease.
Methods
Search strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations and guidelines. The search strategy queried the electronic databases PubMed, Embase and the Cochrane Central Register of Controlled Trials from 1 January 2000 to 31 December 2020. The search terms were: ventricular free-wall rupture OR ventricular pseudoaneurysm AND percutaneous treatment; ventricular septal rupture OR ventricular septal defect AND percutaneous treatment; papillary muscle rupture AND percutaneous treatment. The reference lists of previous systematic reviews and of included studies were assessed to ensure no additional publications were missed.
Inclusion criteria
A qualitative assessment of the percutaneous treatment of ventricular FWR and PMR was undertaken, given the limited experience reported in the literature. The inclusion criteria for the qualitative assessment were all-English language papers (including case reports), with sufficient outcome data reported. Conference abstracts, review articles, editorials and letters were excluded. Papers for inclusion were independently identified and verified (MG, DR, LM and FT).
A quantitative assessment of the primary percutaneous treatment of post-myocardial infarction (PMI) VSD was undertaken. Eligibility for inclusion in this quantitative component were all studies that assessed outcomes of patients undergoing percutaneous treatment of post-infarct VSD as the first treatment. To ensure sufficient centre experience, papers were only included if more than five cases were reported. Only English language papers were analyzed. If centres reported outcomes of overlapping patient series, the most complete, contemporary series was analyzed. Conference abstracts, case reports, editorials, reviews, letters and expert opinion pieces were excluded. Studies detailing the outcomes of the percutaneous treatment of failed previous surgical treatment in post-MI VSD were excluded, as were studies detailing congenital VSD repair. Article identification and inclusion were performed independently by three authors (CDF, PM and MM) and, discussed until consensus was reached.
Data extraction
Data was extracted from the reviewed text, tables and figures. Data was extracted independently by the authors and, any discrepancies were reviewed and discussed until consensus was reached. For the qualitative synthesis, recorded data included: demographic data, acuity of presentation, procedural success, early and follow-up mortality. For the quantitative synthesis, recorded parameters were: number of cases in series, demographic data, follow-up duration, acuity treatment, presence of cardiogenic shock at time of treatment, procedural success, presence and significance of residual trans-septal shunt at discharge from hospital, requirement for subsequent procedures (percutaneous or surgical), timing of treatment and early mortality.
Statistical methods
Meta-analysis of post-operative observations was performed using R (R version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria). For publications reporting continuous variables, central tendency with median and range values, the mean and standard deviation were estimated using calculations described by Wan et al. (5). Given the range of publication dates and operation techniques, significant between-study heterogeneity was anticipated thus, a random effects model was used in all cases. Heterogeneity was assessed using the I2 test statistic and determination of prediction intervals. Low heterogeneity was denoted by I2<50%, moderate heterogeneity by 50%≤I2<75% and high heterogeneity by I2≥75%. For continuous variables, the restricted maximum likelihood estimator was used to calculate heterogeneity variance. Binary data was logit transformed and assessment of heterogeneity was conducted using the DerSimonian-Laird estimator. A Knapp-Hartung adjustment was used to calculate the confidence interval of the pooled outcome effect.
Quality assessment
For the quantitative analysis, study quality was assessed using the risk of bias in non-randomised studies of interventions (ROBINS-i) tool (6). Study quality was independently assessed by two investigators (CDF and PM) until consensus was reached.
Assessment of bias and individual study influence
Publication bias was assessed through visual inspection and statistical analysis of funnel plots. Statistical analysis of funnel plot asymmetry was conducted using Egger’s regression test. Regression analysis of plot asymmetry was only conducted in instances where more than 10 studies were analysed. In the event of significant publication bias, Duval and Tweedie’s trim-and-fill method was applied to correct for publication and small study bias. Leave-one-out sensitivity analysis was conducted to determine the significance of study influence.
Results
The search strategy revealed 3,051 unique articles that underwent title and abstract review, which led to 2,905 studies being excluded. Of the remaining 146 studies, 15 studies were included in the qualitative synthesis of the percutaneous management of PMR (7-21), 4 were included in the qualitative analysis of the percutaneous management of LV FWR (22-25), 7 studies defined the outcomes of the percutaneous management of LV pseudoaneurysm (15,26-41) and 25 were included in the quantitative meta-analysis of the primary percutaneous management of post-MI VSD (42-66) (Table 1) (Figure S1).
Table 1
| Study | Location | Study design | Study focus | Patients (n) | Males (n) | Age ± SD | Study duration | Follow-up years (± SD) |
|---|---|---|---|---|---|---|---|---|
| Maltais, 2009 | Quebec, Canada | Retrospective single centre | Percutaneous Advances of PMI-VSD Closure | 12 | – | 71.3±7.7 | 12 | – |
| Trivedi, 2015 | Marseille, France | Retrospective multicentre | Sequential Management of PMI-VSDs | 8 | – | 75.5±7.0 | 6 | – |
| Bialkowski, 2007 | Zabrze, Poland | Retrospective single centre | Transcatheter Closure of PMI-VSDs Using Amplatzer Devices | 17 | 13 | 66.3±8.1 | 6 | – |
| Aggarwal, 2018 | Kerala, India | Retrospective single centre | ASD Occluder Usage in PMI-VSR | 21 | 15 | 66.4±5.9 | 14 | – |
| Ahmed, 2007 | Auckland, New Zealand | Retrospective single centre | Percutaneous Closure of PMI-VSD | 4 | 2 | 73±4.7 | 3 | 1.95±1.2 |
| Assenza, 2013 | Boston, USA | Retrospective single centre | Transcatheter closure of PMI-VSR | 12 | 8 | 68±6 | 20 | – |
| Martinez, 2007 | Minnesota, USA | Retrospective single centre | Transcatheter Closure of Ischemic and Post-Traumatic VSRs | 4 | 2 | 73±16.5 | – | 4.5 |
| Demkow, 2005 | Warsaw, Poland | Retrospective single centre | Primary Transcatheter Closure of PMI-VSDs with Amplatzer Septal Occluder | 11 | 9 | 67.8±8.9 | 6 | 3.07±1.6 |
| Egbe, 2015 | Minnesota, USA | Retrospective single centre | Transcatheter Closure of PMI, Iatrogenic and Postoperative VSDs | 18 | 7 | 69±11 | 14 | 7.3±7 |
| Nie, 2017 | Taipei, Taiwan | Retrospective multicentre | Transcatheter Device Closure of PMI-VSD | 7 | 5 | 74.4±7.8 | 3 | – |
| Premchand, 2017 | Hyderabad, India | Retrospective single centre | Percutaneous Closure of PMI-VSR | 7 | 2 | 58.3±9.8 | 10 | – |
| Goldsweig, 2018 | Connecticut, USA | Retrospective multicentre | VSR Complicating AMI | 84 | – | – | 9 | – |
| Hamilton, 2017 | Bristol, UK | Retrospective single centre | The In Vivo Morphology of PMI-VSD and Implications for Closure | 16 | – | – | 10 | – |
| Heiberh, 2014 | Aarhus, Denmark | Retrospective single centre | Long-Term Outcome after Transcatheter Closure of PMI-VSR | 9 | 4 | 75.1±8.4 | 13 | 4.6±4.4 |
| Landzberg, 1998 | Boston, USA | Prospective single centre | Transcatheter Management of PMI-VSRs | 7 | – | – | 8 | |
| Sabiniewicz, 2017 | Warsaw, Poland | Retrospective multicentre | Percutaneous Closure of PMI-VSDs | 20 | 11 | 70.2±9.5 | 13 | 1.32±2.6 |
| Sathananthan, 2013 | Auckland, New Zealand | Retrospective single centre | Evolution in PMI-VSD Management | 7 | – | – | 20 | – |
| Rao, 2015 | Hyderabad, India | Prospective single centre | Transcatheter closure of PMI-VSR | 6 | 5 | 60±3.7 | 3 | |
| Szkutnik, 2003 | Zabrze, Poland | Prospective single centre | PMI-VSD Closure with Amplatzer Occluders | 6 | 5 | 59.7±8.1 | – | 9.1±5.8 |
| Tai, 2018 | Hengyang, China | Retrospective multicentre | Management and Outcome of VSR Complicating AMI | 20 | – | – | 10 | |
| Tang, 2015 | Hunan, China | Retrospective single centre | Non-Surgical Repair of VSR After AMI | 11 | 4 | 65.25±7.9 | 7 | 2.55±1.7 |
| Thiele, 2009 | Leipzig, Germany | Prospective single centre | Immediate Primary Transcatheter Closure of PMI-VSD | 29 | 13 | 69±8.9 | 5 | 3.84 |
| Xu, 2014 | Shanghai, China | Retrospective multicentre | Percutaneous Closure of PMI-VSDs | 42 | 4 | – | 4 | 2.25±1.1 |
| Zhang, 2017 | Harbin, China | Retrospective multicentre | Percutaneous Transcatheter Closure of PMI-VSDs: Outcomes and Follow-Up | 15 | 6 | 63.1±7.3 | 12 | 7.6±0.9 |
| Zhu, 2013 | Shenyang, China | Retrospective multicentre | Outcomes of Transcatheter Closure of VSD in Combination with PCI in Patients with VSD Complicating AMI | 35 | 18 | 63.5±5.5 | 10 | 4.42 |
MI, myocardial infarction; VSD, ventricular septal defect; PMI, post-myocardial infarction; ASD, atrial septal defect; VSR, ventricular septal rupture; PCI, percutaneous coronary intervention.
Qualitative synthesis of the percutaneous treatment of mechanical complication of ischaemic heart disease
Papillary muscle rupture (PMR)
Fifteen studies focused on novel treatments of PMR, including 42 patients with an age range of 51–85 (7-21). 24 patients presented in cardiogenic shock (8-15,17,19-21), 22 with pulmonary oedema (8-12,14-21) and 23 required mechanical circulatory support (8-13,15-21) (Table 2). On presentation, 33 patients underwent percutaneous coronary intervention (7-11,14-21), with 27 diagnosed with a STEMI (8-11,13,14,16,19-21) and 13 NSTEMI (12,13,15,17,21). All patients underwent percutaneous edge-to-edge repair of mitral valve using the MitraClip system, with successful deployment. Residual moderate-severe MR was reported in 13 patients (8,13,20,21). Only two manuscripts reported complications of stroke and heart block requiring permanent pacemaker insertion (9,18). There was one reported 30-day mortality (13) and four deaths at time of follow-up (8,13,21) (Table 3).
Table 2
| Author | Year | Patients (n) | Age [range] | Cardiogenic shock [%] | Pulmonary Oedema [%] | MCS [%] | STEMI [%] | NSTEMI [%] | PTCA [%] | PMR [%] | Not PMR [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilge | 2013 | 1 | 60 | 1 [100] | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 1 [100] | 0 |
| Adamo | 2014 | 5 | 72 [62–78] | 4 [80] | 4 [80] | 4a [80] | 5 [100] | 0 | 5 [100] | 0 | 5 [100] |
| Bilge | 2014 | 1 | 73 | – | – | – | – | – | 1 [100] | 1 [100] | 0 |
| Horstkotte | 2014 | 1 | 75 | 1 [100] | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 1 [100] | 0 |
| Wolff | 2014 | 1 | 68 | 1 [100] | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 1 [100] | 0 |
| Bahlmann | 2015 | 1 | 77 | 1 [100] | 1 [100] | 1a [100] | 0 | 1 [100] | – | 1 [100] | 0 |
| Estevez-Loureiro | 2015 | 5 | 68 [51–76] | 3 [60] | – | 3a [60] | 3 [60] | 2 [40] | – | 0 | 5 [100] |
| Rodriguez | 2015 | 1 | 76 | 1 [100] | 1 [100] | – | 1 [100] | 0 | 1 [100] | 0 | 1 [100] |
| Alkhouli | 2017 | 1 | 77 | 1 [100] | 1 [100] | 1b | 0 | 1 [100] | 1 [100] | 0 | 1 [100] |
| Tarsia | 2016 | 1 | 65 | – | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 0 | 1 [100] |
| Valle | 2017 | 1 | 84 | 1 [100] | 1 [100] | – | 0 | 1 [100] | 1 [100] | 1 [100] | 0 |
| Yasin | 2018 | 1 | 68 | 0 | 1 [100] | 1c [100] | – | – | 1 [100] | 1 [100] | 0 |
| Komatsu | 2019 | 1 | 55 | 1 [100] | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 1 [100] | 0 |
| Papadopoulos | 2019 | 1 | 85 | 1 [100] | 1 [100] | 1a [100] | 1 [100] | 0 | 1 [100] | 1 [100] | 0 |
| Haberman | 2019 | 20 | 68 [53–85] | 8 [40] | 7 [35] | 7a [35] | 12 [60] | 8 [40] | 17 [85] | 0 | 20 [100] |
| Total# | – | 42 | 72 [51–85] | 24 [60] | 22 [61] | 23 [59] | 27 [69] | 13 [38] | 33 [92] | 9 [21] | 33 [79] |
#, percentage calculation for summary totals only include patients in studies directly reporting outcome of interest. a, intra-aortic balloon pump; b, Impella device; c, ECMO, extracorporeal membrane oxygenation; MCS, mechanical circulatory support; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; PTCA, percutaneous transcatheter coronary angioplasty; PMR, papillary muscle rupture; MI, myocardial infarction.
Table 3
| Author | Year | Patients (n) | ICU (days) [range] | Discharge (day) [range] | Complication | 30 day-Mortality [%] | NYHA | Grade MR residual [n] | Mortality at follow up [%] | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| Bilge | 2013 | 1 | – | 9 | Stroke | 0 | – | – | 0 | – |
| Adamo | 2014 | 5 | 8 [7–10] | 27 [13–60] | - | 0 | II | 1+ [1], 2+ [1], 3+ [1] | 2 | Septic shock heart failure |
| Bilge | 2014 | 1 | – | – | – | – | – | – | – | – |
| Horstkotte | 2014 | 1 | 7 | 12 | 0 | 0 | – | 1+ [1] | 0 | – |
| Wolff | 2014 | 1 | – | 14 | – | 0 | II | 1+ [1] | 0 | – |
| Bahlmann | 2015 | 1 | 8 | 16 | 0 | – | – | – | – | – |
| Estevez-Loureiro | 2015 | 5 | – | – | – | 1 [20] | II | 1+ [2], 2+ [3] | 1 | MOFs |
| Rodriguez | 2015 | 1 | – | – | – | – | – | – | – | – |
| Alkhouli | 2017 | 1 | 6 | – | – | 0 | I | – | 0 | – |
| Tarsia | 2016 | 1 | 7 | – | 0 | 0 | I | 0 | 0 | – |
| Valle | 2017 | 1 | 4 | – | – | 0 | II | 1+ [1] | 0 | – |
| Yasin | 2018 | 1 | – | – | BAV II | 0 | – | 1+ [1] | 0 | – |
| Komatsu | 2019 | 1 | 0 | 0 | 0 | – | ||||
| Papadopoulos | 2019 | 1 | – | 19 | 0 | 0 | II | 2+ [1] | 0 | – |
| Haberman | 2019 | 20 | – | – | – | 0 | NA | 1+ [12], 2+ [7] | 1 | Sudden death |
| Total# | – | 42 | 7 [4–10] | 15 [9–60] | – | 1 [3] | – | 4 [10] |
#, percentage calculation for summary totals only include patients in studies directly reporting outcome of interest. MI, myocardial infarction; ICU, Intensive care unit; NYHA, New York Heart Association heart failure grade; MOF, multi-organ failure.
Left ventricular free wall rupture (FWR)
Four manuscripts reviewed the management of FWR, including 26 patients with an age range of 51–87 years old (22-25). All patients presented with cardiac tamponade, with 16 patients in concurrent cardiogenic shock, of which, eight required mechanical circulatory support (22-25). Of these, three patients suffered a STEMI (22,23,25) and 19 underwent percutaneous coronary intervention (22,24,25) (Table 4). Due to high-risk conditions, all FWR patients were treated with fibrin-glue injection. Complications occurred in two patients, with one requiring surgical repair and another suffering from acute respiratory distress syndrome (ARDS) and sepsis (22,24). However, 30-day mortality was high, with 16 early deaths attributed to septic shock, acute rupture, re-rupture or non-cardiac causation, and 17 at time of follow-up (22,24) (Table 5).
Table 4
| Author | Year | Patients (n) | Age [range] | Cardiogenic shock [%] | Tamponade [%] | MCS [%] | STEMI [%] | NSTEMI [%] | PTCA [%] |
|---|---|---|---|---|---|---|---|---|---|
| Murata | 2000 | 2 | 69 [56–81] | 2 [100] | 2 [100] | 1 [50] | 1 [50] | 0 | 2 [100] |
| Joho | 2002 | 1 | 82 | 0 | 1 [100] | 0 | 1 [100] | 0 | 0 |
| Terashima | 2008 | 22 | 74 [51–87] | 13 [59] | 22 [100] | 6 | – | – | 16 [72.7] |
| Suzuki | 2019 | 1 | 65 | 1 [100] | 1 [100] | 1 [100] | 1 [100] | – | 1 [100] |
MI, myocardial infarction; MCS, mechanical circulatory support; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; PTCA, percutaneous transcatheter coronary angioplasty.
Table 5
| Author | Year | Patients (n) | Discharge [day] | Complication | 30-day mortality [%] | NYHA | Residual effusion/aneurysm | Mortality at follow up (%) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| Murata | 2000 | 2 | 20 [10–30] | Sepsis, ARDS | 1 [50] | – | 0 | 50 | Septic shock |
| Joho | 2002 | 1 | 14 | – | 0 | – | 0 | 0 | |
| Terashima | 2008 | 22 | – | Required surgical repair (1 patient) | 15 [68] | 1 | 0 | 73 | Acute rupture (13 patients), re-rupture (2 patients), non-cardiac (1 patient) |
| Suzuki | 2019 | 1 | 62 | – | 0 | – | 0 | 0 |
LV, left ventricular; NYHA, New York Heart Association, heart failure grade.
Ventricular pseudoaneurysm
Seventeen studies described techniques for ventricular pseudoaneurysm management, including 19 patients with an age range of 47–85 (15,26-41). Two patients presented with cardiogenic shock (15,39), eight with dyspnoea (15,26,28,31,33,34,38,40) and one required mechanical circulatory support (39). Six patients underwent subsequent percutaneous coronary intervention (33,34,36,37,39,41) and 12 were diagnosed with a STEMI (15,26,27,31-38,41) (Table 6). Due to significant comorbidities, including previous cardiac surgery, COPD, diabetes and previous CVA, all patients underwent percutaneous repair with a closure device. A residual pseudoaneurysm was reported in one patient following attempted closure (34). There were no early deaths and only one mortality at follow-up, due to recurrent pulmonary embolus (27) (Table 7).
Table 6
| Author | Year | Patients (n) | Age [range] | Cardiogenic shock [%] | Dyspnea [%] | MCS [%] | STEMI [%] | NSTEMI [%] | PTCA [%] |
|---|---|---|---|---|---|---|---|---|---|
| Clift | 2004 | 1 | 60 | – | 1 [100] | – | 1 [100] | - | – |
| Harrison | 2007 | 1 | 47 | – | – | – | 1 [100] | - | – |
| Acharya | 2012 | 1 | 49 | 0 | 1 [100] | 0 | – | - | 0 |
| Kar | 2012 | 1 | 67 | 0 | 0 | 0 | – | - | – |
| Subban | 2012 | 1 | 54 | 0 | 0 | 0 | – | - | – |
| Acar | 2013 | 1 | 48 | 0 | 1 [100] | 0 | 1 [100] | – | – |
| Alkhouli | 2015 | 1 | 71 | 1 [100] | 1 [100] | 0 | 1 [100] | 0 | 0 |
| Moriarty | 2015 | 1 | 72 | 0 | 0 | 0 | 1 [100] | 0 | 0 |
| Singh | 2015 | 1 | 82 | 0 | 1 [100] | 0 | 1 [100] | 0 | 1 [100] |
| Madan | 2016 | 1 | 60 | 0 | 1 [100] | 0 | 1 [100] | 0 | 1 [100] |
| Nogueria | 2016 | 1 | 81 | 0 | 0 | 0 | 1 [100] | 0 | 0 |
| Yudi | 2017 | 1 | 79 | 0 | 0 | 0 | 1 [100] | 0 | 1 [100] |
| Pavani | 2018 | 1 | 65 | 0 | 0 | 0 | 1 [100] | 0 | 1 [100] |
| Tang | 2019 | 1 | 79 | 0 | 1 [100] | 0 | 1 [100] | 0 | 0 |
| Bing | 2020 | 3 | 78 [70–85] | 1 [33] | – | 1 [33] | – | – | 1 [33] |
| Cavalcanti | 2020 | 1 | 84 | 0 | 1 [100] | – | – | – | – |
| Gonzalez | 2020 | 1 | 64 | – | – | – | 1 [100] | – | 1 [100] |
| Total# | 19 | 70 [47–85] | 2 [13] | 8 [57] | 1 [8] | 12 [100] | 0 | 6 [23] |
#, percentage calculation for summary totals only include patients in studies directly reporting outcome of interest. MI, myocardial infarction; MCS, mechanical circulatory support; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; PTCA, percutaneous transcatheter coronary angioplasty.
Table 7
| Author | Year | Patients (n) | Discharge (day) [range] | 30 day-Mortality (%) | NYHA | Residual pseudoaneurysm (n) | Mortality at follow up [%] | Cause of death |
|---|---|---|---|---|---|---|---|---|
| Clift | 2004 | 1 | – | – | I | 0 | 0 | – |
| Harrison | 2007 | 1 | 0 | – | – | 1 | Recurrent PE | |
| Archarya | 2012 | 1 | – | 0 | – | – | 0 | – |
| Kar | 2012 | 1 | – | 0 | – | – | 0 | – |
| Subban | 2012 | 1 | – | – | – | – | – | – |
| Acar | 2013 | 1 | 5 | – | – | – | – | – |
| Alkhouli | 2015 | 1 | – | 0 | – | – | 0 | – |
| Moriarty | 2015 | 1 | – | 0 | – | 0 | 0 | – |
| Singh | 2015 | 1 | 3 | 0 | I | 0 | 0 | – |
| Madan | 2016 | 1 | – | – | II | 1 [100] | 0 | – |
| Nogueria | 2016 | 1 | – | 0 | – | – | 0 | – |
| Yudi | 2017 | 1 | – | – | – | – | – | – |
| Pavani | 2018 | 1 | – | – | – | – | – | – |
| Tang | 2019 | 1 | 7 | 0 | – | 0 | 0 | – |
| Bing | 2020 | 3 | – | – | – | – | 0 | – |
| Cavalcanti | 2020 | 1 | 5 | – | I | – | 0 | – |
| Gonzalez | 2020 | 1 | 7 | – | – | – | – | – |
| Total# | 19 | 5 [3–7] | 0 | – | 1 [20] | 1 [7] | – |
#, percentage calculation for summary totals only include patients in studies directly reporting outcome of interest. LV, left ventricular; NYHA, New York Heart Association, heart failure grade; PE, pulmonary embolus..
Meta-analysis of post-infarct VSD
Study quality and risk of bias assessment
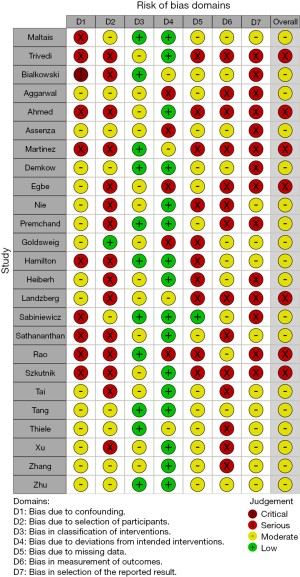
The overall risk of bias was moderate to severe for all 25 included studies (Figure 1). There were 7 studies (43,47,51,53,58,59,63) graded with an overall severe risk and 18 studies (42,44-46,48-50,52,54-57,60-62,64-66) graded at overall moderate risk, of bias. The most significant risks of bias were identified in confounding, selection bias and selection of reported outcomes.
Demographic data
The total number of patients in the analysis was 428. Average age was reliably reported in 19 studies (42-47,50,52-56,58,59,61-63,65,66) with a pooled mean age of 67.6 years-old [95% confidence interval (CI): 65.4–69.9, I2=80.3%]. Eighteen studies (42-47,50,53-56,58-62,65,66) provided data regarding patient gender. In these studies, 48.5% of patients (133 of 274 patients) were male. Duration of data collection was clearly reported in 25 studies (42-52,54-58,60-66), which collected data over a mean duration of 9.5±4.9 years. The median number of annual cases in the studies reporting data was 1.5 [inter-quartile range (IQR) 1.0] cases per year (Table 1).
Mortality outcomes
All 25 studies reported outcomes on mortality. There were 174 deaths in 428 patients. The proportion of patients experiencing early mortality was 37.5% (95% CI: 29.0–46.8%, I2=65%). On influence analysis, no studies were found to have excessive influence on outcome or between study heterogeneity. There was a suggestion of publication bias with an over-representation of patients experiencing low mortality on visual inspection of funnel plot (Figure S2A). Funnel plot asymmetry was statistically significant on Egger’s test (P=0.037). Duval and Tweedie trim-and-fill method to correct for possible publication bias revealed an estimated proportion of patients experiencing early mortality of 53.3% (95% CI: 42.7–63.5%, I2=79.5%), with an additional 10 imputed studies (Figure S2B). There was no identifiable impact of centre annual caseload on early mortality on meta-regression (P=0.654).
Procedural success
Procedural success was reliably reported in 21 studies (42-47,50,52-56,58-66). There were 43 failed procedures in 314 patients. The proportion of failed procedures was 15.9% (95% CI: 12.0–20.8%, I2=0%). There were no overly influential studies on influence analysis. There was no evidence of publication bias on visual inspection of funnel plots (Egger’s regression test, P=0.41). Presence of post-procedural shunt was reported in 15 studies (43,45,46,50,53,54,56-58,61-66). 21 out of 225 patients demonstrated a moderate or severe shunt at the time of discharge. The proportion of patients with a moderate or severe shunt was 13.8% (95% CI: 9.3–19.8%, I2=0%). There were no overly influential studies. There was asymmetry evident on visual inspection of forest plots which was found to be significant on Egger’s regression test (P=0.001) (Figure S3A). Trim-and-fill suggested the proportion of patients with a significant trans-septal shunt would be 18.2% (95% CI: 11.6–27.3%) after correction of publication bias, with 6 additional imputed studies (trim-and-fill funnel plot, Figure S3B).
Further procedures
Twenty studies (42-47,50,52-56,58-65) reported outcomes on the requirement of further procedures (both surgical and percutaneous), following percutaneous closure of post-infarct VSD. There were 46 patients out of 279 who required follow-up procedures. The proportion of patients requiring follow-up procedures was 19.3% (95% CI: 13.2–27.3%, I2=38.6%). There were no overly influential studies. There was evidence of asymmetry on visual inspection of funnel plot confirmed on Egger’s regression analysis (P=0.014) (Figure S4A). Trim-and-fill analysis suggests the proportion of patients requiring further procedures is 26.5% (95% CI: 18.2–36.7%) after correction of small study bias, with 7 additional imputed studies (trim-and-fill funnel plot, Figure S4B).
Impact of acuity on survival
The reported pooled average time for VSD diagnosis post-acute MI in 8 studies was 2.90 days (95% CI: 1.97–3.82 days, I2=62.9%) (42-44,54,61,62,64). The time to VSD repair after diagnosis was reported in 17 studies as 22.0 days (95% CI: 12.4–31.6 days, I2=99.4%) (43,44,46,49,50,52-56,59-62,64,66).
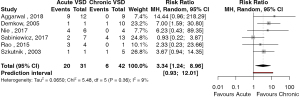
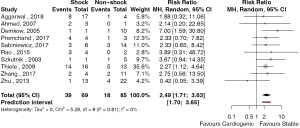
The outcomes of patient survival based on acuity of presentation was reported in 6 studies (42,46,54,56,58,59). There were 20 deaths in 31 patients presenting with acute post-infarct VSD and 6 deaths in 42 patients presenting with chronic post-infarct VSD. The risk ratio (RR) of mortality for patients with acute versus chronic post-infarct VSD was 3.34 (95% CI: 1.2–9.0, P=0.026, I2=8.8%) (Figure 2). The study by Sabiniewicz (56) was found to have an influential effect on the risk associated with the treatment of acute post-infarct VSDs. Exclusion of this study increased the RR to 5.07 (95% CI: 2.51–10.21, P<0.001, I2=0%). Given the small study number, publication bias was unable to be formally assessed. Ten studies reported outcomes based on whether patients presented in cardiogenic shock. There were 39 deaths in 69 patients who presented with cardiogenic shock and, 18 deaths in 85 patients who presented in a stable condition. The RR of mortality between patients with cardiogenic shock compared to stable patients was 2.49 (95% CI: 1.71–3.63, P<0.001, I2=0%) (Figure 3). There were no significantly influential studies, nor evidence of publication bias.
Discussion
The mechanical complications of ischemic heart disease are rare but devastating to the patients that suffer them. Acutely presenting patients often present critically unwell with significant morbidity and mortality associated with conventional surgical repair. This review has highlighted the emerging field of the percutaneous management of mechanical complications of IHD which, although having been practiced for several decades, remains in its infancy.
The early results of the treatment of PMR have encouraging results, with a low early mortality of 1 patient of the 42 reported cases. This low mortality contrasts with the reported early mortality for surgical intervention for PMR, which is 12.5–20% (67-69). However, the reported mortality for percutaneous mitral valve repair is almost certainly substantially underestimated through small study bias. The Japanese and American registry data reports the predominant mode of surgical intervention for PMR was mitral valve replacement in 79.8–90% (68,69), giving a reliable result. This review has demonstrated a significant minority (31%) of patients treated with percutaneous mitral repair were left with moderate or severe MR in a highly selectively reported group of patients. This review also demonstrates that the catastrophic and difficult to manage complications of LV FWR and LV free wall pseudoaneurysm can be managed in certain circumstances with percutaneous techniques. Although, this data is still very much case report based and thus, future study is certainly warranted.
The overall mortality of percutaneous repair of PMI-VSD reported in this study (37.5%) is similar to surgical outcomes of post-infarct VSD from various national registry data, ranging from 33.0–42.9% (70-72). This is despite a significant proportion of patients treated with primary percutaneous intervention being of prohibitive surgical risk. Only one study used percutaneous treatment as the preferred treatment, over surgical repair, for all patients presenting with PMI-VSD (62). However, the assessment of publication bias suggests overall mortality from percutaneous intervention is likely to be underestimated due to the presence of small study bias. The corrected estimated mortality was higher than reported surgical outcomes at 53.3%.
This manuscript highlights the increased risk that patients are at when they require early surgery. Acute surgery has been demonstrated to be high-risk for surgical intervention, for both PMI-VSD and PMR (68-72). Various strategies of prolonging time to surgery have been proposed including, the use of medical support with inotropes and mechanical assist devices, such as intra-aortic balloon pumps and extra-corporeal membrane oxygenation. Attempts have been made to provide extended periods of mechanical support to allow haemodynamic stabilization and an improvement in biochemical parameters prior to intervention. Mortality of patients requiring aggressive management with mechanical support to facilitate delayed surgical repair remains high, although a recent meta-analysis of published literature by Ronco and colleagues (73) assessed the surgical outcomes of 2,440 patients of whom 129 were treated with ECMO support prior to surgical repair. This study suggests a mortality benefit in patients treated with a prolonged period of stabilization using mechanical support compared to those operated on emergently. Currently, this strategy is not recommended to be utilised as a routine intervention for patients with post-infarct VSD who present in extremis although, mechanical cardiac support is recommended as a bridge to urgent repair (74).
Limitations
There are several important limitations with this analysis. All studies are of at least a moderate risk bias due to the small study size, retrospective analysis, absence of randomisation and implicit selection bias. There will be a substantial element of publication bias as well. However, this is an emerging field of technologies that is likely to expand with increased experience of all transcatheter and percutaneous interventions.
Conclusions
Mechanical complications of IHD are becoming an increasingly rare phenomenon due to advances in the management of coronary artery disease however, the associated mortality and morbidity remains high. Although surgical techniques remain the gold standard, we have shown that percutaneous management may be a viable option in certain cases. Further study and accurate reporting are clearly required to determine the true level of risk associated with these techniques, to ensure they are appropriately applied in these rare conditions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol 2010;105:59-63. [Crossref] [PubMed]
- Damluji AA, van Diepen S, Katz JN, et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021;144:e16-35. [Crossref] [PubMed]
- Gong FF, Vaitenas I, Malaisrie SC, et al. Mechanical Complications of Acute Myocardial Infarction: A Review. JAMA Cardiol 2021;6:341-9. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021;12:55-61. [Crossref] [PubMed]
- Bilge M, Alemdar R, Ali S, et al. Percutaneous mitral valve repair with the MitraClip system in a patient with subacute severe mitral regurgitation caused by papillary muscle rupture. Anadolu Kardiyol Derg 2014;14:475-6. [Crossref] [PubMed]
- Adamo M, Curello S, Chiari E, et al. Percutaneous edge-to-edge mitral valve repair for the treatment of acute mitral regurgitation complicating myocardial infarction: A single centre experience. Int J Cardiol 2017;234:53-7. [Crossref] [PubMed]
- Bilge M, Alemdar R, Yasar AS. Successful percutaneous mitral valve repair with the MitraClip system of acute mitral regurgitation due to papillary muscle rupture as complication of acute myocardial infarction. Catheter Cardiovasc Interv 2014;83:E137-40. [Crossref] [PubMed]
- Horstkotte JC, Horstkotte M, Beucher H, et al. Percutaneous mitral valve repair as rescue procedure after post myocardial infarction papillary muscle rupture and acute cardiogenic shock. Clin Res Cardiol 2015;104:275-8. [Crossref] [PubMed]
- Wolff R, Cohen G, Peterson C, et al. MitraClip for papillary muscle rupture in patient with cardiogenic shock. Can J Cardiol 2014;30:1461.e13-4. [Crossref] [PubMed]
- Bahlmann E, Frerker C, Kreidel F, et al. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg 2015;99:e41-2. [Crossref] [PubMed]
- Estévez-Loureiro R, Arzamendi D, Freixa X, et al. Percutaneous Mitral Valve Repair for Acute Mitral Regurgitation After an Acute Myocardial Infarction. J Am Coll Cardiol 2015;66:91-2. [Crossref] [PubMed]
- Rodríguez-Santamarta M, Estévez-Loureiro R, Gualis J, et al. Percutaneous Mitral Valve Repair With Mitraclip® System in a Patient With Acute Mitral Regurgitation After Myocardial Infarction. Rev Esp Cardiol (Engl Ed) 2015;68:259-61. [Crossref] [PubMed]
- Alkhouli M, Wolfe S, Alqahtani F, et al. The Feasibility of Transcatheter Edge-to-Edge Repair in the Management of Acute Severe Ischemic Mitral Regurgitation. JACC Cardiovasc Interv 2017;10:529-31. [Crossref] [PubMed]
- Tarsia G, Smaldone C, Costantino MF. Effective percutaneous "edge-to-edge" mitral valve repair with mitraclip in a patient with acute post-MI regurgitation not related to papillary muscle rupture. Catheter Cardiovasc Interv 2016;88:1177-80. [Crossref] [PubMed]
- Valle JA, Miyasaka RL, Carroll JD. Acute Mitral Regurgitation Secondary to Papillary Muscle Tear: Is Transcatheter Edge-to-Edge Mitral Valve Repair a New Paradigm? Circ Cardiovasc Interv 2017;10:e005050. [Crossref] [PubMed]
- Yasin M, Nanjundappa A, Annie FH, et al. Use of MitraClip for Postmyocardial Infarction Mitral Regurgitation Secondary to Papillary Muscle Dysfunction. Cureus 2018;10:e3065. [Crossref] [PubMed]
- Komatsu I, Cohen EA, Cohen GN, et al. Transcatheter Mitral Valve Edge-to-Edge Repair with the New MitraClip XTR System for Acute Mitral Regurgitation Caused by Papillary Muscle Rupture. Can J Cardiol 2019;35:1604.e5-7. [Crossref] [PubMed]
- Papadopoulos K, Chrissoheris M, Nikolaou I, et al. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep 2019;3:ytz001. [Crossref] [PubMed]
- Haberman D, Taramasso M, Czarnecki A, et al. Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study. Eur J Heart Fail 2019;21:1161-4. [Crossref] [PubMed]
- Murata H, Masuo M, Yoshimoto H, et al. Oozing type cardiac rupture repaired with percutaneous injection of fibrin-glue into the pericardial space: case report. Jpn Circ J 2000;64:312-5. [Crossref] [PubMed]
- Joho S, Asanoi H, Sakabe M, et al. Long-term usefulness of percutaneous intrapericardial fibrin-glue fixation therapy for oozing type of left ventricular free wall rupture: a case report. Circ J 2002;66:705-6. [Crossref] [PubMed]
- Terashima M, Fujiwara S, Yaginuma GY, et al. Outcome of percutaneous intrapericardial fibrin-glue injection therapy for left ventricular free wall rupture secondary to acute myocardial infarction. Am J Cardiol 2008;101:419-21. [Crossref] [PubMed]
- Suzuki A, Ishikawa J, Nemoto Y, et al. Acute left ventricular free wall rupture treated by percutaneous intra-pericardial fibrin-glue infusion. J Echocardiogr 2019;17:106-8. [Crossref] [PubMed]
- Clift P, Thorne S, de Giovanni J. Percutaneous device closure of a pseudoaneurysm of the left ventricular wall. Heart 2004;90:e62. [Crossref] [PubMed]
- Harrison W, Ruygrok PN, Greaves S, et al. Percutaneous closure of left ventricular free wall rupture with associated false aneurysm to prevent cardioembolic stroke. Heart Lung Circ 2008;17:250-3. [Crossref] [PubMed]
- Acharya D, Nagaraj H, Misra VK. Transcatheter closure of left ventricular pseudoaneurysm. J Invasive Cardiol 2012;24:E111-4. [PubMed]
- Kar B, Gholkar G, Gregoric ID, et al. Percutaneous closure of a left ventricular pseudoaneurysm in a high-risk surgical candidate. Tex Heart Inst J 2012;39:680-2. [PubMed]
- Subban V, Kurian VM, Ajit MS, et al. Hybrid trans-apical device closure of left ventricular pseudoaneurysm under trans-oesophageal echocardiographic guidance. Heart Lung Circ 2012;21:734-6. [Crossref] [PubMed]
- Acar Z, Korkmaz L, Parlar A, et al. Percutaneous Closure of Huge Left Ventricular Pseudoaneurysm by Atrial Septal Occluder Device. International Journal of Cardiology 2013. doi: 10.1016/S0167-5273(13)70360-7. [Crossref]
- Moriarty J, Harris TJ, Vorobiof G, et al. Direct Percutaneous Repair of Left Ventricular Pseudoaneurysm via Transthoracic Deployment of a Ventricular Septal Defect Closure Device. Tex Heart Inst J 2015;42:362-6. [Crossref] [PubMed]
- Singh A, Kliger C, Ruiz CE. Novel approach for the percutaneous treatment of left ventricular pseudoaneurysms. Catheter Cardiovasc Interv 2015;85:1092-6. [Crossref] [PubMed]
- Madan T, Juneja M, Raval A, et al. Transcatheter device closure of pseudoaneurysms of the left ventricular wall: An emerging therapeutic option. Rev Port Cardiol 2016;35:115.e1-5. [Crossref] [PubMed]
- Afonso Nogueira M, Fiarresga A, de Sousa L, et al. Percutaneous closure of a giant left ventricular wall pseudoaneurysm: Anterograde approach with a double snare technique. Rev Port Cardiol 2016;35:441.e1-4. [Crossref] [PubMed]
- Yudi MB, Love B, Nadir A, et al. Percutaneous Closure of Left Ventricular Pseudoaneursym With Septal Occluder Device and Coils: A Multimodality Imaging Approach. JACC Cardiovasc Interv 2017;10:e159-e161. [Crossref] [PubMed]
- Pavani M, Scrocca I, Meliga E, et al. Percutaneous Closure of a Left Ventricular Pseudoaneurysm Causing Recurrent Embolization, With Cerebral Protection and Extracorporeal Membrane Oxygenation Support. JACC Cardiovasc Interv 2018;11:e7-e10. [Crossref] [PubMed]
- Tang L, Tang JJ, Hu XQ, et al. Transcatheter Closure of Complex Post-Myocardial Infarction Left Ventricular Pseudoaneurysm and Unique Post-Traumatic Right Ventricular Pseudoaneurysm. Int Heart J 2019;60:998-1002. [Crossref] [PubMed]
- Bing R, Behan MWH, Pessotto R, et al. Percutaneous Repair of Ventricular Ruptures. JACC Case Rep 2020;2:341-6. [Crossref] [PubMed]
- Cavalcanti LRP, Sá MPBO, Escorel Neto AC, et al. Percutaneous closure of left ventricular pseudoaneurysm in a patient with concomitant true left ventricular aneurysm. J Card Surg 2021;36:2113-6. [Crossref] [PubMed]
- Fernandez Gonzalez L, Blanco Mata R, Garcia San Román K, et al. Percutaneous Closure of Perforated Ventricular Septal Pseudoaneurysm With "Cribriform" Amplatzer Device Using 3D Modeling. JACC Cardiovasc Interv 2020;13:e211-4. [Crossref] [PubMed]
- Aggarwal M, Natarajan K, Vijayakumar M, et al. Primary transcatheter closure of post-myocardial infarction ventricular septal rupture using amplatzer atrial septal occlusion device: A study from tertiary care in South India. Indian Heart J 2018;70:519-27. [Crossref] [PubMed]
- Ahmed J, Ruygrok PN, Wilson NJ, et al. Percutaneous closure of post-myocardial infarction ventricular septal defects: a single centre experience. Heart Lung Circ 2008;17:119-23. [Crossref] [PubMed]
- Assenza GE, McElhinney DB, Valente AM, et al. Transcatheter closure of post-myocardial infarction ventricular septal rupture. Circ Cardiovasc Interv 2013;6:59-67. [Crossref] [PubMed]
- Bialkowski J, Szkutnik M, Kusa J, et al. Transcatheter closure of postinfarction ventricular septal defects using Amplatzer devices. Rev Esp Cardiol 2007;60:548-51. [Crossref] [PubMed]
- Demkow M, Ruzyllo W, Kepka C, et al. Primary transcatheter closure of postinfarction ventricular septal defects with the Amplatzer septal occluder- immediate results and up-to 5 years follow-up. EuroIntervention 2005;1:43-7. [PubMed]
- Egbe AC, Poterucha JT, Rihal CS, et al. Transcatheter closure of postmyocardial infarction, iatrogenic, and postoperative ventricular septal defects: The Mayo Clinic experience. Catheter Cardiovasc Interv 2015;86:1264-70. [Crossref] [PubMed]
- Goldsweig AM, Wang Y, Forrest JK, et al. Ventricular septal rupture complicating acute myocardial infarction: Incidence, treatment, and outcomes among medicare beneficiaries 1999-2014. Catheter Cardiovasc Interv 2018;92:1104-15. [Crossref] [PubMed]
- Hamilton MCK, Rodrigues JCL, Martin RP, et al. The In Vivo Morphology of Post-Infarct Ventricular Septal Defect and the Implications for Closure. JACC Cardiovasc Interv 2017;10:1233-43. [Crossref] [PubMed]
- Heiberg J, Hjortdal VE, Nielsen-Kudsk JE. Long-term outcome after transcatheter closure of postinfarction ventricular septal rupture. J Interv Cardiol 2014;27:509-15. [Crossref] [PubMed]
- Landzberg MJ, Lock JE. Transcatheter management of ventricular septal rupture after myocardial infarction. Semin Thorac Cardiovasc Surg 1998;10:128-32. [Crossref] [PubMed]
- Maltais S, Ibrahim R, Basmadjian AJ, et al. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg 2009;87:687-92. [Crossref] [PubMed]
- Martinez MW, Mookadam F, Sun Y, et al. Transcatheter closure of ischemic and post-traumatic ventricular septal ruptures. Catheter Cardiovasc Interv 2007;69:403-7. [Crossref] [PubMed]
- Nie YL, Lin MC, Lin WW, et al. Transcatheter device closure of postmyocardial infarction ventricular septal defect. J Chin Med Assoc 2017;80:34-8. [Crossref] [PubMed]
- Premchand RK, Garipalli R, Padmanabhan TN, et al. Percutaneous closure of post-myocardial infarction ventricular septal rupture - A single centre experience. Indian Heart J 2017;69:S24-7. [Crossref] [PubMed]
- Sabiniewicz R, Huczek Z, Zbroński K, et al. Percutaneous Closure of Post-Infarction Ventricular Septal Defects-An Over Decade-long Experience. J Interv Cardiol 2017;30:63-71. [Crossref] [PubMed]
- Sathananthan J, Ruygrok P. Evolution in the management of postinfarct ventricular septal defects from surgical to percutaneous approach: a single-center experience. J Invasive Cardiol 2013;25:339-43. [PubMed]
- Seshagiri Rao D, Patnaik AN, Barik R, et al. Transcatheter closure of postinfarction ventricular septal rupture. Journal of Indian College of Cardiology 2015;5:220-7. [Crossref]
- Szkutnik M, Bialkowski J, Kusa J, et al. Postinfarction ventricular septal defect closure with Amplatzer occluders. Eur J Cardiothorac Surg 2003;23:323-7. [Crossref] [PubMed]
- Tai S, Tang JJ, Tang L, et al. Management and Outcome of Ventricular Septal Rupture Complicating Acute Myocardial Infarction: What Is New in the Era of Percutaneous Intervention? Cardiology 2018;141:226-32. [Crossref] [PubMed]
- Tang L, Fang Z, Hu X, et al. Non-surgical repair of ventricular septal rupture after acute myocardial infarction. Int J Cardiol 2015;185:328-32. [Crossref] [PubMed]
- Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J 2009;30:81-8. [Crossref] [PubMed]
- Trivedi KR, Aldebert P, Riberi A, et al. Sequential management of post-myocardial infarction ventricular septal defects. Arch Cardiovasc Dis 2015;108:321-30. [Crossref] [PubMed]
- Xu XD, Liu SX, Liu X, et al. Percutaneous closure of postinfarct muscular ventricular septal defects: a multicenter study in China. J Cardiol 2014;64:285-9. [Crossref] [PubMed]
- Zhang R, Sun Y, Sun M, et al. In-Hospital Outcomes and Long-Term Follow-Up after Percutaneous Transcatheter Closure of Postinfarction Ventricular Septal Defects. Biomed Res Int 2017;2017:7971027. [Crossref] [PubMed]
- Zhu XY, Qin YW, Han YL, et al. Long-term efficacy of transcatheter closure of ventricular septal defect in combination with percutaneous coronary intervention in patients with ventricular septal defect complicating acute myocardial infarction: a multicentre study. EuroIntervention 2013;8:1270-6. [Crossref] [PubMed]
- Sultan I, Aranda-Michel E, Gleason TG, et al. Mitral valve surgery for acute papillary muscle rupture. J Card Surg 2018;33:484-8. [Crossref] [PubMed]
- Kilic A, Sultan I, Chu D, et al. Mitral Valve Surgery for Papillary Muscle Rupture: Outcomes in 1342 Patients From The Society of Thoracic Surgeons Database. Ann Thorac Surg 2020;110:1975-81. [Crossref] [PubMed]
- Fujita T, Yamamoto H, Kobayashi J, et al. Mitral valve surgery for ischemic papillary muscle rupture: outcomes from the Japan cardiovascular surgery database. Gen Thorac Cardiovasc Surg 2020;68:1439-46. [Crossref] [PubMed]
- Dimagli A, Guida G, Sinha S, et al. Surgical outcomes of post-infarct ventricular septal defect repair: Insights from the UK national adult cardiac surgery audit database. J Card Surg 2022;37:843-52. [Crossref] [PubMed]
- Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2012;94:436-43; discussion 443-4. [Crossref] [PubMed]
- Sakaguchi G, Miyata H, Motomura N, et al. Surgical Repair of Post-Infarction Ventricular Septal Defect- Findings From a Japanese National Database. Circ J 2019;83:2229-35. [Crossref] [PubMed]
- Ronco D, Matteucci M, Ravaux JM, et al. Mechanical Circulatory Support as a Bridge to Definitive Treatment in Post-Infarction Ventricular Septal Rupture. JACC Cardiovasc Interv 2021;14:1053-66. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]






