Procedural and clinical outcomes of transcatheter aortic valve replacement in bicuspid aortic valve patients: a systematic review and meta-analysis
Introduction
Bicuspid aortic valve (BAV) anatomy affects up to 2% of the population and is an important risk factor for the development of aortic stenosis (AS) (1). Development of moderate to severe AS occurs in 12–37% of patients with BAV and AS may manifest up to 20 years earlier in these patients compared to those with a tricuspid aortic valve (TAV) (2,3). 20% of patients with AS over the age of 80 have BAV anatomy, and surgical aortic valve replacement (SAVR) is the current mainstay of treatment.
Traditionally, transcatheter aortic valve replacement (TAVR) has been reserved for patients ineligible or unsuitable for SAVR. Recent data has shown, however, that TAVR produces comparable or potentially favorable outcomes compared to SAVR (4-7).
Currently, BAV remains a relative contraindication for TAVR due to concerns associated with suboptimal valve-in-valve anatomy (8,9). Such concerns include increased annular ellipticity and asymmetric calcification, potentially resulting in inadequate fixation of the prosthetic valve, leading to an increased risk of paravalvular leak (PVL) or prosthesis migration (8,9). In contrast, SAVR avoids these potential issues via resection of the diseased valve and fixation of the prosthesis (5,10). Clinical trials regarding TAVR have therefore excluded patients with BAV and as such, BAV remains outside TAVR guidelines (4,5,11-13).
However, recently increasing off-label use of TAVR for BAV stenosis and improved valve technology have shown promising outcomes, comparable both to TAVR in TAV stenosis and to SAVR in BAV stenosis (14-18). These results primarily stem from high-risk patients ineligible for SAVR, but there remains optimism that TAVR could be a viable or preferred treatment for all patients with BAV stenosis. Currently, long-term data regarding efficacy and outcomes of TAVR in BAV patients is scarce. This study aims to investigate the rapidly growing body of literature on both short- and mid-term outcomes of TAVR in BAV patients.
Methods
Literature search strategy
The systematic review was conducted under the direction of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) (19). An electronic keyword and medical subject heading (MeSH) search was performed on Medline, Scopus, Embase, Cochrane and EBM Reviews Databases with the following search terms: (“transcatheter aortic valve replacement” OR “transcatheter valve replacement” OR “TAVR” OR “transcatheter aortic valve implantation” OR “TAVI” OR “percutaneous aortic valve implantation” OR “PAVR”) AND (“bicuspid” OR “bicuspid aortic valve” OR “BAV”). Studies containing search terms in the title or abstract published between January 2002 and September 2021 were included for screening and duplications were removed. All references and published systematic reviews were manually screened for additional studies.
Eligibility criteria
Studies were screened for inclusion and exclusion criteria independently by two authors (CHJ Chen, H Jiang). Discrepancies were discussed until an agreement was reached. Studies fulfilling the following criteria were included in this study: (I) studies including BAV patients undergoing TAVR; (II) adult (>18 years of age) human studies with more than ten patients; (III) studies reporting survival outcomes at 30-days, one or two years; (IV) English studies. Studies were excluded if the inclusion criteria were not met or if it satisfied one of the following exclusion criteria: (I) case reports, editorials, reviews, commentaries and conference abstracts; (II) studies with patients undergoing TAVR as a redo procedure; (III) studies with patients with endocarditis. Where studies contained overlapping data, preference was given to the study with the longest follow-up period.
Data extraction and critical appraisal
Data was extracted from text, figures and tables by three authors independently (CHJ Chen, H Jiang, O Martin). Endpoints were derived from Valve Academic Research Consortium-2 (VARC-2) consensus document in conjunction with commonly reported outcomes in reviewed studies (20). Reported endpoints with a total number of patients less than 10% of the total study population were excluded from the analysis. The primary endpoint was mortality and secondary endpoints include post-procedural and clinical outcomes. Quality assessment was performed using a modified schema designed for assessing case series, developed by the Institute of Health Economics (Alberta, Canada) (Table S1) (21). Study quality was determined via assessment of study objective, design, population, intervention, outcome measures, statistical analysis, appropriateness of results and conclusions and competing interests. Studies were determined to be of low quality if they satisfied fewer than 10 criteria, of moderate quality if they satisfied 10–12 criteria and of high quality if they satisfied more than 12 criteria.
Statistical analysis
Meta-analyses of means and proportions were performed using the continuous and binary Dersimonian-Laird random effects models, respectively. Pooled means are presented as a mean value (95% confidence interval). Pooled proportions are presented as a percentage (95% confidence interval). Data reported as median and interquartile range was assumed to be skewed and converted into mean ± standard deviation using the Box-Cox method as described by McGrath et al. (22). Heterogeneity assessment across the studies was performed using the I2 statistic. I2 values of 0–49%, 50–74% and 75–100% were deemed to represent low, moderate and high heterogeneity, respectively. Statistical analysis was performed on OpenMeta[Analyst] (Center for Evidence-based Medicine, Brown University, USA) (23). P values <0.05 were considered statistically significant.
Results
Study details
A total of 5,064 records were identified following a literature search, of which 22 studies were included in this study after exclusion (Figure 1). The majority of the data was sourced from the United States (five studies), Mainland China (four studies), Italy (three studies), Poland (three studies) and France (three studies) (Table 1). Other countries/region involved in the study included Korea, Taiwan, Denmark, Germany, Israel, The Netherlands, Switzerland, Japan and Canada. Seven studies were found to be of high quality, 13 studies of medium quality and two studies of low quality (Table 1).
Table 1
| Study, publication year | Study type | Patient recruitment | Data source | Country/region | Comparison | N | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Husso (15), 2021 | Cohort | Retrospective | FinnValve Registry | Finland | SAVR | 103 | High |
| Sun (24), 2021 | Cohort | Prospective | First Affiliated Hospital of Air Force Medical University | China | TAV | 51 | Medium |
| Gorla (25), 2021 | Cohort | Retrospective | 3 academic centres | Italy | Prosthetic type | 56 | Medium |
| Jung (26), 2021 | Cohort | Prospective | Seoul National University Hospital | Korea | TAV | 19 | Medium |
| Kumar (27), 2021 | Cohort | Retrospective | Knight Cardiovascular Institute | United States | BAV morphology | 30 | Low |
| Tsai (16), 2021 | Cross-sectional | Retrospective | Cheng-Hsin General Hospital | Taiwan | SAVR | 48 | Low |
| Kochman (28), 2020 | Case series | Retrospective | Polish Registry | Poland | n/a | 24 | High |
| Pineda (29), 2020 | Cohort | Retrospective | Duke aortic valve disease database | United States | TAV | 50 | Medium |
| Yoon (30)*, 2020 | Cohort | Prospective* | International Bicuspid Aortic Valve Stenosis Registry | International | BAV calcification | 1,034 | Medium |
| Fu (31), 2020 | Cohort | Retrospective | Beijing Fuwai Hospital | China | BAV morphology | 44 | High |
| Waksman (32), 2020 | Case series | Retrospective | LRT Trial | United States | TAV | 61 | Medium |
| Fan (33), 2020 | Cohort | Prospective | Second Affiliated Hospital of Zhejiang University | China | n/a | 83 | Medium |
| Aalaei-Andabili (34), 2018 | Cohort | Prospective | University of Florida Health Care Centre | United States | TAV | 32 | High |
| Liao (18), 2018 | Cohort | Prospective | West China Hospital, Sichuan | China | TAV | 87 | Medium |
| De Biase (35), 2018 | Cohort | Prospective | Groupe Cardiovasculaire Interventionel, Clinique Pasteur | France | TAV | 83 | Medium |
| Djordjevic (36), 2017 | Case series | Retrospective | Deutsches Herzzentrum Berlin | Germany | TAV | 33 | Medium |
| Watanabe (37), 2015 | Cohort | Prospective | Teikyo University Hospital | Japan | n/a | 11 | High |
| Costopoulos (38), 2014 | Cohort | Retrospective | San Rafaelle Scientific Institute | Italy | TAV | 21 | Medium |
| Clinical Institute S. Ambrogio | |||||||
| Kochman (39), 2014 | Cohort | Retrospective | 5 academic centres | Poland | TAV | 28 | High |
| Hayashida (40), 2013 | Cohort | Prospective | Institut Cardiovasculaire, Paris | France | TAV | 21 | High |
| Himbert (41), 2012 | Case series | Retrospective | Bichat-Claude Bernard Hospital, Paris | France | TAV | 15 | Medium |
| Wijesinghe (42), 2010 | Case series | Retrospective | St. Paul’s Hospital | Canada | n/a | 11 | Medium |
| Quebec Heart and Lung Institute | |||||||
| Hamilton Health Sciences Centre |
*, the study by Yoon et al. [2020] drew from the International Bicuspid Aortic Valve Stenosis Registry in which patients were recruited both retrospectively and prospectively. BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; SAVR, surgical aortic valve replacement; N, number of patients with bicuspid valves included in each study; LRT, low-risk TAVR; TAVR, transcatheter aortic valve replacement; n/a, not available.
Baseline characteristics
A total of 1,945 patients with BAV stenosis undergoing TAVR from 22 studies were included in the meta-analysis. Of these patients, 59.1% (95% CI: 56.2–62.0%; I2=12%) were male. The mean age in this cohort was 74.1 (95% CI: 72.4–75.9; I2=94%) years. The Society of Thoracic Surgeons-Predicted Risk of Mortality (STS-PROM) was 5.39 (95% CI: 4.45–6.34; I2=98%) and the proportion of heart failure patients with function within New York Heart Association (NYHA) class III or IV was 71.8% (95% CI: 63.4–80.2%; I2=93%). General echocardiographic findings of the patient population included a left ventricular ejection fraction (LVEF) of 52.2% (95% CI: 50.0–54.5%; I2=91%), a mean aortic gradient of 54 mmHg (95% CI: 51–58 mmHg; I2=91%), an aortic valve area of 0.64 cm2 (95% CI: 0.60–0.69 cm2; I2=91%), an aortic annulus area of 530 mm2 (95% CI: 490–580 mm2; I2=91%), a mean aortic annulus diameter of 25.7 mm (95% CI: 24.5–26.9 mm; I2=96%), and an ascending aortic size of 74.1 mm (95% CI: 72.4–75.9 mm; I2=91%) (Table 2, Figure S1). All P values were statistically significant.
Table 2
| Characteristic | Patients [studies], n | Weighted pooled estimate [95% CI] | Heterogeneity I2 (%) |
|---|---|---|---|
| Age (years) | 1,945 [22] | 74.1 [72.4–75.9] | 94 |
| Male sex (%) | 1,844 [20] | 59.1 [56.2–62.0] | 12 |
| STS-PROM score | 1,861 [18] | 5.39 [4.45–6.34] | 98 |
| NYHA class III/IV (%) | 1,743 [16] | 71.8 [63.4–80.2] | 93 |
| LVEF (%) | 1,741 [19] | 52.2 [50.0–54.5] | 91 |
| Mean aortic gradient (mmHg) | 1,728 [18] | 54 [51–58] | 91 |
| Aortic valve area (cm2) | 1,492 [14] | 0.64 [0.60–0.69] | 91 |
| Aortic annulus area (mm2) | 298 [6] | 530 [490–580] | 91 |
| Mean aortic annulus diameter (mm) | 403 [12] | 25.7 [24.5–26.9] | 96 |
| Ascending aortic size (mm) | 1,510 [14] | 74.1 [72.4–75.9] | 91 |
CI, confidence interval; n, number of patients; STS-PROM, Society of Thoracic Surgeons-Predicted Risk of Mortality; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction.
Procedures
The route of access was reported in 18 studies, and 91.8% of procedures were transfemoral. The most common devices used were the CoreValve (Medtronic, Minneapolis, Minnesota, USA) and Evolut R (Medtronic) (15,18,25-27,29,30,32-41), used in 17 studies, and the SAPIEN 3 (Edwards Lifesciences, Irvine, California, USA) and SAPIEN XT valves (Edwards Lifesciences), used in 13 studies (15,26,30,32-40,42). The Lotus EDGE (Boston Scientific, Marlborough, Massachusetts, USA) was used in six studies (15,25,26,28,33,35) and the VenusA-valve (Venus MedTech, Hangzhou, China) was used in four studies from Mainland China (18,24,31,33). Other less commonly used valves included the Arcuate neo valve (Boston Scientific), the VITAFLOW aortic valve system (Microport, Shanghai, China), the TaurusOne transcatheter aortic valve system (Peijia Medical, Suzhou, China) and the Portico system (Abbott Structural Heart, St. Paul, Minnesota, USA).
Post-procedural outcomes
The overall device success rate was 87.5% (95% CI: 82.4–92.7%; I2=72%). Moderate to severe PVL was seen in 3.7% (95% CI: 2.2–5.3%; I2=46%) of patients. Echocardiographic findings following TAVR included a mean aortic gradient of 11.2 mmHg (95% CI: 9.8–12.6 mmHg; I2=96%), an effective orifice area of 1.70 cm2 (95% CI: 1.67–1.73 cm2; I2=91%) and a LVEF of 55.2% (95% CI: 53.0–57.5%; I2=81%). Device migration was reported in 2.5% (95% CI: 0.5–4.5%; I2=0%) of procedures (Table 3, Figure S2). All P values were statistically significant.
Table 3
| Outcome | Patients [studies], n | Weighted pooled estimate [95% CI] | Heterogeneity I2 (%) |
|---|---|---|---|
| Device success (%) | 483 [11] | 87.5 [82.4–92.7] | 72 |
| Moderate/severe PVR (%) | 1,806 [18] | 3.7 [2.2–5.3] | 46 |
| Mean aortic gradient (mmHg) | 1,661 [18] | 11.2 [9.8–12.6] | 96 |
| Effective orifice area (cm2)* | 1,077 [3] | 1.70 [1.67–1.73] | 91 |
| LVEF (%) | 1,354 [10] | 55.2 [53.0–57.5] | 81 |
| Device migration (n) | 223 [7] | 2.5 [0.5–4.5] | 0 |
*, Djordjevic et al. was excluded following sensitivity analysis. CI, confidence Interval; LVEF, left ventricular ejection fraction; PVR, pulmonary vascular resistance.
Clinical outcomes
The mean hospital stay was 7.68 days (95% CI: 6.17–9.19 days; I2=99%). New permanent pacemaker insertion (PPI) was required in 11.8% (95% CI: 7.9–15.8%; I2=87%) of procedures. The most common clinical complication was major bleeding (3.5%; 95% CI: 1.8–5.2%; I2=36%), followed by major vascular complications (2.5%; 95% CI: 1.2–3.9%; I2=41%), stroke (2.3%; 95% CI: 1.6–3.0%; I2=0%), acute kidney injury (2.1%; 95% CI: 1.0–3.1%; I2=48%) and coronary obstruction (0.1%; 95% CI: 0.1–0.2%; I2=0%). Conversion to open surgery was required in 1.0% of procedures (95% CI; 0.5–1.5%; I2=0%) (Table 4, Figure S3). The P value for coronary obstruction was 0.294. All other P values were statistically significant.
Table 4
| Outcome | Patients [studies], n | Weighted pooled estimate [95% CI] | Heterogeneity I2 (%) |
|---|---|---|---|
| Length of hospital stay (days) | 465 [10] | 7.68 [6.17–9.19] | 99 |
| Coronary obstruction (%) | 1,531 [14] | 0.1 [0.1–0.2] | 0 |
| Conversion to surgery (%) | 1,448 [13] | 1.0 [0.5–1.5] | 0 |
| Major vascular complication (%) | 1,542 [12] | 2.5 [1.2–3.9] | 41 |
| Major bleeding (%) | 1,471 [13] | 3.5 [1.8–5.2] | 36 |
| Stroke (%) | 1,872 [19] | 2.3 [1.6–3.0] | 0 |
| Acute kidney injury* (%) | 1,355 [9] | 2.1 [1.0–3.1] | 48 |
| New PPI (%) | 1,824 [18] | 11.8 [7.9–15.8] | 87 |
*, Pineda et al. was excluded following sensitivity analysis. CI, confidence interval; PPI, permanent pacemaker insertion.
All-cause mortality
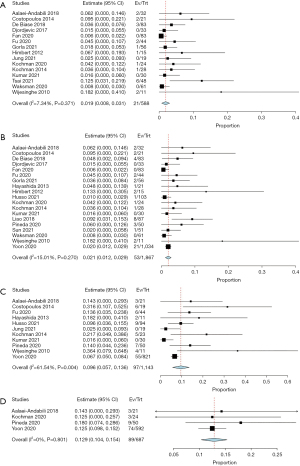
The mean in-hospital mortality of BAV patients following TAVR was 1.9% (95% CI: 0.8–3.1%; I2=7%). The mortality at 30 days and one-year post-procedure was 2.1% (95% CI: 1.2–2.9%; I2=15%) and 9.6% (95% CI: 5.7–13.6%; I2=62%), respectively. Mean mortality at two years post-procedure was 12.9% (95% CI: 10.4–15.4%; I2=0%). Two papers reported mortality rates of 11.0% and 15.8% at their respective follow-ups of 2.1±1.6 and 2.86±1.47 years (Table 5, Figure 2), respectively (15,16). All P values were statistically significant.
Table 5
| Length of time post-operation | Patients [studies], n | Weighted pooled estimate [95% CI] | Heterogeneity I2 (%) |
|---|---|---|---|
| In-hospital (%) | 588 [15] | 1.9 [0.8–3.1] | 7 |
| 30-day (%) | 1,867 [19] | 2.1 [1.2–2.9] | 15 |
| 1-year (%) | 1,143 [11] | 9.6 [5.7–13.6] | 62 |
| 2-year (%) | 635 [4] | 12.9 [10.4–15.4] | 0 |
CI, confidence interval.
Discussion
BAV has traditionally been a contraindication to TAVR, due to complications arising from the abnormal anatomy of the aortic valve, which is not excised and remains in situ following TAVR (30). As a result, BAV patients have been excluded from landmark TAVR randomized controlled trials (RCTs) and its efficacy and safety profile in BAV patients remain uncertain (43,44). SAVR has been the mainstay of treatment for BAV stenosis; however, with the increasing use of TAVR in BAV patients, data comparing the outcomes of TAVR and SAVR in BAV patients is becoming more available (15,16,45). Husso et al. conducted a cohort study of 75 propensity score-matched patients and found that 30-day and two-year mortality of BAV patients undergoing SAVR were 5.3% and 18.7%, respectively, and the difference compared to TAVR was not statistically significant (15). Elbadawi et al. also found in a cross-sectional study of over 1,000 patients that there was no significant difference in in-hospital mortality between SAVR and TAVR for BAV patients (45). Interestingly, a recent cross-sectional study of 48 BAV patients found that although there was no difference in survival rates between BAV patients undergoing TAVR and SAVR, functional recovery (as defined by patient-reported maximum activity level) after six months was greater in SAVR patients compared to TAVR (16). These studies show promising short- and mid-term results for TAVR as an alternative to SAVR in BAV patients, and long-term follow-up studies investigating both morbidity and mortality are warranted to further assess the safety and efficacy of TAVR in BAV patients.
The current study found that BAV patients undergoing TAVR had a 30-day and one-year overall mortality of 2.1% and 9.6% respectively. Included studies that reported the highest 30-day mortality were from 2010 to 2014, while studies that reported the lowest 30-day mortality were from 2020 to 2021 (15,24,27,38,41,42). This trend was also seen in one-year mortality results, where the three studies that reported the highest one-year mortality were from 2010 to 2014, while the three studies with the lowest one-year mortality were from 202 to 2021 (26,27,30,38,39,42). This may suggest an improved safety profile of TAVR in BAV patients, as centers are increasingly incorporating TAVR as an alternative or even preferred treatment for BAV stenosis. Two-year mortality was found to be 12.9% in the current patient cohort, and this is the only systematic review to our knowledge that reports aggregated two-year mortality in BAV patients undergoing TAVR.
This systematic review found low rates of procedural and clinical outcomes. Device success rate (87.5%) reported in this study is comparable with previously published systematic reviews, which range from 85.8% to 95.2% (46-49). Post-procedural mean aortic gradient (11.2 mmHg) was also comparable with previously published gradients, which range from 6.0 to 16.0 mmHg (49,50). The rate of moderate to severe PVL (3.7%) was found to be lower in this cohort compared to previously published cohorts, which range from 8.8% to 12.2% (46-48,50). It is interesting to note that 82.4% of patients from this systematic review are from studies published after 2020, suggesting that increased experience with TAVR in BAV may play a role in mitigating post-procedural PVL.
The risk of requiring a new PPI (11.8%) was highest following TAVR in BAV patients, although there was significant heterogeneity within the reported studies. Major bleeding (3.5%), major vascular complications (2.5%) and acute kidney injury (2.1%) were the next most common complications in this patient cohort. This is consistent with previously published systematic reviews, which reported a new PPI rate of 12.2–18.5%, a major bleeding rate of 4.2–20.0%, an acute kidney injury risk of 2.04–6.50%, and a major vascular complication rate of 1.3–8.5% (46,48-56). Following sensitivity analysis, Pineda et al. was excluded from meta-analysis of AKI due to its significantly high rate, which was not representative of the current patient cohort. This may be attributable to the high rate of comorbidities in their patient cohort compared to other studies in the systematic review, including 84% of patients with hypertension and 46% of patients with diabetes mellitus (15,16,29,30,32). The rate of coronary obstruction (0.1%) reported in this systematic review was lower than previously published rates (0.5–1.6%) (49,52,53,56). Yoon et al. reported no coronary obstructions in 1,034 patients, and while this significantly impacted the data following sensitivity analysis, the study contributed more than half of the patients included in this systematic review and was included for meta-analysis (30). The same study also reported low rates of stroke and conversion to open surgery (30). Nevertheless, studies comparing these post-procedural outcomes to those of SAVR are warranted to further assess the complication risk of TAVR in BAV stenosis versus standard treatment.
Several large, multicenter studies have found no differences in clinical outcomes and survival between BAV and TAV patients undergoing TAVR, with rates similar to those reported in the current study (14,57,58). Yoon et al. compared short- and mid-term mortality between 546 pairs of propensity score-matched TAV and BAV patients undergoing TAVR and found that there were no significant differences in 30-day, one-year or two-year mortality (14). Interestingly, the same study found that while BAV patients undergoing TAVR using new-generation devices (Sapien 3, Lotus, Evolut R) do not differ in PVL, device failure, second valve implantation or conversion to surgery compared to TAV patients, patients using old-generation devices (Sapien XT, CoreValve) experienced higher rates of these complications (14). Similar results were found in another 2017 prospective cohort study of 400 patients, which reported higher rates of procedural complications (device failure, second valve implantation, moderate/severe aortic regurgitation) 30-day mortality, aortic regurgitation and major vascular complications when using old-generation devices, regardless of valve anatomy (59). Despite this, recent unpublished data suggests that there are still areas of concern for the use of TAVR in BAV stenosis, as higher rates of PVL, annular rupture and cerebral ischemic events were reported compared to TAV (60). Results from the current study include both old- and new-generation devices and are comparable to morbidity and mortality results from TAVR studies in TAV patients (14,59). Taken together, this data shows increasing promise for the role of TAVR as a treatment option in BAV stenosis.
Limitations and future directions
There are several limitations to this study. BAV patients in this systematic review were studied as a single cohort, and subgroup analyses were not performed between different groups of BAV patients. There was significant heterogeneity within the baseline characteristics of the study population (Figure S1). However, following sensitivity analysis, no single study was found to significantly affect overall study outcomes. Previous studies have identified several procedural and patient specific variables that may impact the mortality and clinical outcomes in BAV patients undergoing TAVR. These include BAV morphology and degree of calcification, device type/generation, radiological features and surgical approach (14,30,59,61-63). Additionally, long-term follow-up data was not included in this study, due to the lack of available studies in the current literature. Currently, several RCTs (NCT03163329, NCT02541877) and a long-term follow-up study (NCT0365424) are running, and results from these studies will add valuable information to the existing body of literature regarding the viability of TAVR as a treatment modality for BAV stenosis.
Conclusions
This evaluation of the progress of TAVR for BAV stenosis demonstrates that it is associated with promising short- and mid-term morbidity and mortality outcomes. Recent TAVR developments are in the right direction for it to become a viable alternative to SAVR. Long-term outcomes remain unclear for TAVR in BAV and randomized trials with long-term follow-up will provide greater insight into its safety and efficacy.
Acknowledgments
The authors would like to acknowledge the following contributors: (I) Dr. David Tian for his contribution to the direction of the study; (II) Librarian Linda Mulheron of Westmead Hospital Library for her contribution to the systematic review; (III) Consultant statistician Jim Matthews for his contribution to the meta-analysis.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braverman AC, Güven H, Beardslee MA, et al. The bicuspid aortic valve. Curr Probl Cardiol 2005;30:470-522. [Crossref] [PubMed]
- Masri A, Svensson LG, Griffin BP, et al. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart 2017;103:1323-30. [Crossref] [PubMed]
- Shen M, Tastet L, Capoulade R, et al. Effect of age and aortic valve anatomy on calcification and haemodynamic severity of aortic stenosis. Heart 2017;103:32-9. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. [Crossref] [PubMed]
- Zegdi R, Ciobotaru V, Noghin M, et al. Is it reasonable to treat all calcified stenotic aortic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol 2008;51:579-84. [Crossref] [PubMed]
- Kochman J, Rymuza B, Huczek Z. Transcatheter aortic valve replacement in bicuspid aortic valve disease. Curr Opin Cardiol 2015;30:594-602. [Crossref] [PubMed]
- Van Praet KM, van Kampen A, Kofler M, et al. Minimally invasive surgical aortic valve replacement: The RALT approach. J Card Surg 2020;35:2341-6. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-e643. Erratum in: Circulation 2014 Jun 10;129(23):e651; Circulation. 2014 Sep 23;130(13):e120. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200-54. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol 2017;69:2579-89. [Crossref] [PubMed]
- Husso A, Airaksinen J, Juvonen T, et al. Transcatheter and surgical aortic valve replacement in patients with bicuspid aortic valve. Clin Res Cardiol 2021;110:429-39. [Crossref] [PubMed]
- Tsai HY, Lin YS, Wu IC, et al. Major adverse cardiac events and functional capacity in patients at intermediate risk undergoing transcatheter versus surgical aortic valve replacement for aortic stenosis with bicuspid valves. J Card Surg 2021;36:828-33. [Crossref] [PubMed]
- Elbadawi A, Mahmoud AA, Mahmoud K, et al. Temporal Trends and Outcomes of Elective Thoracic Aortic Repair and Acute Aortic Syndromes in Bicuspid Aortic Valves: Insights from a National Database. Cardiol Ther 2021;10:531-45. [Crossref] [PubMed]
- Liao YB, Li YJ, Xiong TY, et al. Comparison of procedural, clinical and valve performance results of transcatheter aortic valve replacement in patients with bicuspid versus tricuspid aortic stenosis. Int J Cardiol 2018;254:69-74. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg 2012;42:S45-60. [Crossref] [PubMed]
- IHE. Quality Appraisal of Case Series Checklist 2014. Available online: https://www.ihe.ca/publications/ihe-quality-appraisal-checklist-for-case-series-studies
- McGrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. Journal of Statistical Software 2012;49:1-15. [Crossref]
- Sun Q, Wang B, Zhu CJ, Mou F, Yin Z, Wang P, et al. Evaluation of the safety and efficacy of transcatheter aortic valve replacement with domestic prostheses for patients with severely stenotic bicuspid aortic valve. Zhonghua Xin Xue Guan Bing Za Zhi 2021;49:250-6. [PubMed]
- Gorla R, Casenghi M, Finotello A, et al. Outcome of transcatheter aortic valve replacement in bicuspid aortic valve stenosis with new-generation devices. Interact Cardiovasc Thorac Surg 2021;32:20-8. [Crossref] [PubMed]
- Jung JH, Kim HK, Park JB, et al. Progression of ascending aortopathy may not occur after transcatheter aortic valve replacement in severe bicuspid aortic stenosis. Korean J Intern Med 2021;36:332-41. [Crossref] [PubMed]
- Kumar K, Simpson TF, Akhavein R, et al. Hemodynamic and Conduction System Outcomes in Sievers Type 0 and Sievers Type 1 Bicuspid Aortic Valves Post Transcatheter Aortic Valve Replacement. Structural Heart 2021;5:287-94. [Crossref]
- Kochman J, Zbroński K, Kołtowski Ł, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis utilizing the next-generation fully retrievable and repositionable valve system: mid-term results from a prospective multicentre registry. Clin Res Cardiol 2020;109:570-80. [Crossref] [PubMed]
- Pineda AM, Rymer J, Wang A, et al. Transcatheter aortic valve replacement for patients with severe bicuspid aortic stenosis. Am Heart J 2020;224:105-12. [Crossref] [PubMed]
- Yoon SH, Kim WK, Dhoble A, et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:1018-30. [Crossref] [PubMed]
- Fu B, Chen Q, Zhao F, et al. Efficacy and safety of transcatheter aortic valve implantation in patients with severe bicuspid aortic stenosis. Ann Transl Med 2020;8:873. [Crossref] [PubMed]
- Waksman R, Craig PE, Torguson R, et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Bicuspid Aortic Valve Stenosis. JACC Cardiovasc Interv 2020;13:1019-27. [Crossref] [PubMed]
- Fan J, Fang X, Liu C, et al. Brain Injury After Transcatheter Replacement of Bicuspid Versus Tricuspid Aortic Valves. J Am Coll Cardiol 2020;76:2579-90. [Crossref] [PubMed]
- Aalaei-Andabili SH, Beaver TM, Petersen JW, et al. Early and midterm outcomes of transcatheter aortic valve replacement in patients with bicuspid aortic valves. J Card Surg 2018;33:489-96. [Crossref] [PubMed]
- De Biase C, Mastrokostopoulos A, Philippart R, et al. Aortic valve anatomy and outcomes after transcatheter aortic valve implantation in bicuspid aortic valves. Int J Cardiol 2018;266:56-60. [Crossref] [PubMed]
- Djordjevic A, D'Ancona G, Unbehaun A, et al. Transcatheter aortic valve implantation for bicuspid aortic valve stenosis: Acute and intermediate-term outcomes in a high volume institution. Slovenian Medical Journal 2017;86:8-18. [Crossref]
- Watanabe Y, Chevalier B, Hayashida K, et al. Comparison of multislice computed tomography findings between bicuspid and tricuspid aortic valves before and after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2015;86:323-30. [Crossref] [PubMed]
- Costopoulos C, Latib A, Maisano F, et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol 2014;113:1390-3. [Crossref] [PubMed]
- Kochman J, Huczek Z, Scisło P, et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol 2014;114:757-62. [Crossref] [PubMed]
- Hayashida K, Bouvier E, Lefèvre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circ Cardiovasc Interv 2013;6:284-91. [Crossref] [PubMed]
- Himbert D, Pontnau F, Messika-Zeitoun D, et al. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patients with stenotic bicuspid aortic valves. Am J Cardiol 2012;110:877-83. [Crossref] [PubMed]
- Wijesinghe N, Ye J, Rodés-Cabau J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv 2010;3:1122-5. [Crossref] [PubMed]
- Reddy G, Wang Z, Holmes DR. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: Meta analysis of observational studies. Catheter Cardiovasc Interv 2017;89:S200.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Elbadawi A, Saad M, Elgendy IY, et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc Interv 2019;12:1811-22. [Crossref] [PubMed]
- Bob-Manuel T, Heckle MR, Ifedili IA, et al. Outcomes of transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Ann Transl Med 2019;7:102. [Crossref] [PubMed]
- Quintana RA, Monlezun DJ, DaSilva-DeAbreu A, et al. One-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement for Stenotic Bicuspid versus Tricuspid Aortic Valves: A Meta-Analysis and Meta-Regression. J Interv Cardiol 2019;2019:8947204. [Crossref] [PubMed]
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: Systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018;91:975-83. [Crossref] [PubMed]
- Xie X, Shi X, Xun X, et al. Efficacy and Safety of Transcatheter Aortic Valve Implantation for Bicuspid Aortic Valves: A Systematic Review and Meta-Analysis. Ann Thorac Cardiovasc Surg 2016;22:203-15. [Crossref] [PubMed]
- Chan JSK, Singh S, Eriksen P, et al. Transcatheter Aortic Valve Implantation in Bicuspid Aortic Valve with Aortic Stenosis: a Meta-Analysis and Trial Sequential Analysis. Braz J Cardiovasc Surg 2022;37:88-98. [Crossref] [PubMed]
- Kanjanahattakij N, Horn B, Vutthikraivit W, et al. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: A systematic review and meta-analysis. Clin Cardiol 2018;41:896-902. [Crossref] [PubMed]
- Medranda G, Rogers T, Doros G, et al. Transcatheter Aortic Valve Replacement in Low-Risk Bicuspid and Tricuspid Patients - a Systematic Review and Meta-Analysis of Clinical Trials. J Am Coll Cardiol 2021;77:1116. [Crossref]
- Sá MPBO, Simonato M, Van den Eynde J, et al. Balloon versus self-expandable transcatheter aortic valve implantation for bicuspid aortic valve stenosis: A meta-analysis of observational studies. Catheter Cardiovasc Interv 2021;98:E746-57. [Crossref] [PubMed]
- Phan K, Wong S, Phan S, et al. Transcatheter Aortic Valve Implantation (TAVI) in Patients With Bicuspid Aortic Valve Stenosis--Systematic Review and Meta-Analysis. Heart Lung Circ 2015;24:649-59. [Crossref] [PubMed]
- Takagi H, Hari Y, Kawai N, et al. Meta-analysis of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic valves. J Cardiol 2019;74:40-8. [Crossref] [PubMed]
- Ueshima D, Nai Fovino L, Brener SJ, et al. Transcatheter aortic valve replacement for bicuspid aortic valve stenosis with first- and new-generation bioprostheses: A systematic review and meta-analysis. Int J Cardiol 2020;298:76-82. [Crossref] [PubMed]
- Nagaraja V, Suh W, Fischman DL, et al. Transcatheter aortic valve replacement outcomes in bicuspid compared to trileaflet aortic valves. Cardiovasc Revasc Med 2019;20:50-6. [Crossref] [PubMed]
- Makkar RR, Yoon SH, Chakravarty T, et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk. JAMA 2021;326:1034-44. [Crossref] [PubMed]
- Seeger J, Gonska B, Rottbauer W, et al. New generation devices for transfemoral transcatheter aortic valve replacement are superior compared with last generation devices with respect to VARC-2 outcome. Cardiovasc Interv Ther 2018;33:247-55. [Crossref] [PubMed]
- Montalto C, Sticchi A, Crimi G, et al. Outcomes After Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Anatomy: A Systematic Review and Meta-Analysis. JACC Cardiovasc Interv 2021;14:2144-55. [Crossref] [PubMed]
- Ielasi A, Moscarella E, Mangieri A, et al. Procedural and clinical outcomes of type 0 versus type 1 bicuspid aortic valve stenosis undergoing trans-catheter valve replacement with new generation devices: Insight from the BEAT international collaborative registry. Int J Cardiol 2021;325:109-14. [Crossref] [PubMed]
- Weir-McCall JR, Attinger-Toller A, Blanke P, et al. Annular versus supra-annular sizing for transcatheter aortic valve replacement in bicuspid aortic valve disease. J Cardiovasc Comput Tomogr 2020;14:407-13. [Crossref] [PubMed]
- Zhao ZG, Feng Y, Liao YB, et al. Reshaping bicuspid aortic valve stenosis with an hourglass-shaped balloon for transcatheter aortic valve replacement: A pilot study. Catheter Cardiovasc Interv 2020;95:616-23. [Crossref] [PubMed]






