Speaking a common language: the international consensus on bicuspid aortic valve nomenclature and classification
Introduction: why a unified, universal consensus?
Nomenclature refers to the selection of name that is given to a particular structure, abnormality or phenotype, whereas classification refers to the process of arranging or categorizing something according to shared features (1-4). The clinician evaluating the congenital bicuspid aortic valve (BAV) patient must be able to communicate all specific morphological, functional and prognostic aspects of the BAV condition to the patient, other clinicians, surgeons, interventionalists and researchers, in a common language (5). In addition, there are multiple gaps in the knowledge and understanding of the BAV condition (6). For advancing the clinical, biological and genetic understanding of the BAV condition, a common language that incorporates all possible phenotypic expressions, must be articulated among researchers in all clinical and laboratory research disciplines. Multiple nomenclatures and classifications exist for the BAV condition (1-4), and they are as heterogeneous or more than the BAV condition itself (5). For example, the Sievers (7), Schaefer (8) and Kang (9) classifications use multiple letters and numbers to describe different aspects of the BAV and its aortopathy that are not intuitive and also are incomplete (1-4). The use of one of the many classifications for research varies randomly according to authors and institutions, and there are specific terminologies that lead to confusion such as the “true” BAV (6); does it mean that the others are false BAV? These heterogeneous classifications cause confusion in clinical practice; failure to identify phenotypes that may predict outcomes; inability to analyze clinical outcome data in registries, systematic reviews and meta-analysis formats; failure to capture anatomical information critical for surgical aortic valve repair; and hamper identification of phenotypic-genetic associations. This international consensus is an imaging-based, descriptive, simple, but comprehensive, nomenclature and classification system that is based on the English language, rather than on numbers or letters (1-4). In addition, it is based on important available anatomical, clinical, surgical and pathological scientific data. This new nomenclature/classification system represents the combined efforts of international BAV experts including Clinicians (both adult and pediatric), surgeons, interventionalists, pathologists, embryologists, geneticists and imagers [echocardiography, computed tomography (CT) and magnetic resonance (MR) experts] (1-4).
Limitations of the Sievers classification
The Sievers classification (7), based on the presence and number of raphes identified at surgery (i.e., direct surgical visualization), has several shortcomings (Table 1) (1-4): (I) it is not based on imaging, which is the most common method of diagnosing, phenotyping and surveilling the BAV and its aortopathy; (II) it is unable to define all known BAV phenotypes (i.e., does not recognize the partial fusion (forme fruste) BAV and does not recognize fused phenotypes without raphe); (III) it does not recognize the aortopathy phenotypes of a condition (BAV) that is a valvulo-aortopathy; (IV) it lacks assessment of BAV symmetry, which is critical for planning surgical regurgitant-BAV repair (10,11); and (V) it includes as a “BAV”, an incomplete definition of the unicuspid aortic valve morphology (Sievers type 2). Although the morphological spectrum of human congenital aortic valve abnormalities includes unicuspid, bicuspid and quadricuspid aortic valves, their embryologic origins may not necessarily be linked, such that animal models of BAV have displayed all possible BAV phenotypes, quadricuspid valves and pulmonary valve abnormalities, but not unicuspid anatomical forms (12). In addition, the prevalence, age at presentation and prognosis of unicuspid and BAV, are not equivalent (13,14). Furthermore, the anatomic definition of unicuspid aortic valve (one cusp with or without a commissure: unicommissural or acommissural) (15,16) is very different from that of BAV (two cusps, two commissures); therefore, the Sievers classification includes only one (unicommissural) of the two types of unicuspid aortic valves as if it were a subtype of BAV, which is not correct.
Table 1
| Type of limitation | Specific Sievers limitation | International consensus |
|---|---|---|
| Comprehension and retention | Not language-intuitive: types: 0, 1 and 2 | Language-intuitive: types: fused, 2-sinus and partial fusion |
| Unable to define all BAV phenotypes | Type 0 does not differentiate between a fused BAV with no raphe and a 2-sinus BAV | Fused types may have raphe or not; 2-sinus types do not have raphe |
| Lack of pre repair assessment of symmetry | Nonexistent | Fused types require assessment of symmetry for surgical repair planning |
| Lack of recognition of BAV phenotypes | Does not recognize the partial-fusion (forme fruste); does not recognize fused BAV with no raphe | Recognizes partial-fusion (forme fruste); recognizes fused BAV with no raphe, which is different from 2-sinus BAV |
| Lack of recognition of aortopathy phenotypes | Nonexistent | Aorta phenotypes: root, ascending and extended |
| Includes a non-BAV congenital aortic valve abnormality | Type 2 is not BAV, is unicuspid aortic valve | Does not include unicuspid aortic valves |
| Evidence-based | Anatomical surgical pathology only | Imaging, anatomical surgical pathology, surgical-functional pathology, clinical associations |
Nosology of the congenital BAV condition
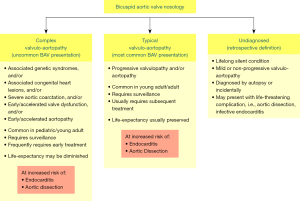
The congenital BAV condition is a valvulo-aortopathy, characterized by significant heterogeneity of its valvular and aortic phenotypic expressions, of its associated disorders, of its complications and of its prognosis (5,6,17-19). To reconcile this clinical and prognostic heterogeneity, the BAV condition is broadly categorized into three clinical-prognostic subgroups (Figure 1). (I) Complex valvulo-aortopathy (1-5,19) is characterized by concomitant or associated disorders that may be clinically and prognostically worse than the BAV condition per se (i.e., Turner syndrome, Loeys-Dietz syndrome, Shone complex and severe aortic coarctation) and/or by early/accelerated valve dysfunction and/or aortopathy, more commonly diagnosed earlier in the pediatric, adolescent and young adult population (20,21). This presentation frequently requires early surgical/invasive treatment and close surveillance. (II) Typical valvulo-aortopathy (1-6) is the most common type, with progressive BAV dysfunction and/or aortic dilatation without other major associated disorders, is more commonly diagnosed in the young adult and adult, requires long-term surveillance and commonly requires subsequent surgical/invasive treatment. Patients with complex-presentation and typical-presentation valvulo-aortopathies are at risk of developing infective endocarditis and aortic dissection (Figure 1), although aortic dissection is extremely rare in young children with BAV and rare in adults without aortic dilatation (6,22). (III) The undiagnosed BAV subgroup (1-4,6) exhibits a lifelong silent condition with mild or non-progressing valvulo-aortopathy that does not manifest clinically but may come to light at autopsy or incidentally by imaging (Figure 1) but may also present with a life-threatening complication with unknown BAV status. Therefore, it commonly represents a retrospective definition. A critical difference between the typical and complex valvulo-aortopathies is the preserved long-term overall life expectancy that is similar to that of the age- and sex-matched general population in patients with the typical valvulo-aortopathy (23), whereas the life expectancy of patients with complex valvulo-aortopathy may be reduced (1-4).
Clinical importance and Fundamentals of imaging assessment of the congenital BAV condition
In order of frequency, the most common complications of the congenital BAV condition are (6,23): (I) the need for aortic valve surgery due to aortic stenosis; (II) ascending thoracic aortic dilatation that may require surgical repair; (III) the need for aortic valve surgery due to aortic regurgitation; (IV) mitral valve prolapse including the anterior leaflet that may require surgery (24); (V) infective endocarditis (25); and (VI) aortic dissection (22).
At the center of the BAV condition is echocardiography, which serves as the first-line imaging modality in 6 major capacities (26): (I) BAV diagnosis; (II) valvular phenotyping; (III) assessment of valvular function (26); (IV) measurement of the thoracic aorta (the expression of BAV aortopathy is dilatation of the thoracic aorta); (V) exclusion of aortic coarctation and other associated congenital lesions (6,20); and (VI) assessment of uncommon but serious complications such as infective endocarditis and aortic dissection. Transthoracic echocardiography (TTE) is the best modality for hemodynamic assessment of valvular dysfunction and the initial modality for assessment of thoracic aorta size, presence of aortic coarctation and other congenital lesions. Transesophageal echocardiography (TEE) may aid in the diagnosis and phenotyping of BAV if it is not well visualized by TTE and, has excellent accuracy for the diagnosis of aortic dissection and infective endocarditis (1-4).
Advanced imaging modalities are also critical in assessing the BAV condition: electrocardiographic (ECG)-gated cardiac CT and ECG-gated MR. These imaging techniques improve diagnostic accuracy and phenotyping of BAV (27,28) and represent the gold standard for measuring the thoracic aorta because they accurately assess aortic diameters that are truly perpendicular to the longitudinal axis of the aorta (1-4). After initial TTE imaging, if any aortic segment cannot be visualized or coarctation cannot be ruled out or any thoracic segment measures ≥45 mm by TTE, then ECG-gated CT angiography or MR angiography, is recommended (29).
Definition of the congenital BAV by the international consensus
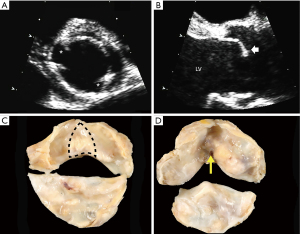
The congenital BAV is most commonly diagnosed by base-of-the-heart, short-axis aortic valve imaging with TTE or TEE or ECG-gated CT or MR, demonstrating the existence of only two commissures delimiting only two valve cusps (Figure 2) (1-4,6,30). On echocardiographic long-axis imaging, systolic doming of the conjoined cusp may be appreciated particularly for right-left coronary cusp fusion (Figure 2), but it is less reliable for identifying other BAV phenotypes. The diagnosis can also be made by direct surgical observation (11,31) and by a pathological examination (32). It is important to recognize that a tricuspid aortic valve that is calcified or rheumatic may present a pattern of acquired (non-congenital) fusion of two cusps that may be difficult to differentiate from congenital BAV. In these cases, surgical inspection and/or pathological examination may identify whether the fusion is congenital or not. In the operating room, the surgeon can define the congenital bicuspid condition by comparing the height of the “pseudocommissure” [the attachment of the “raphe” (pseudo-commissure) at the aortic wall], which is lower within the root compared to the height of the true commissures, whose attachment is higher (Figure 3). Additional gross features can be used on surgical or pathological inspection, such as the angle formed between the fused cusps (obtuse: congenital fusion; acute: acquired fusion) and the cleavage plane on the ventricular aspect of the fused cusps (absent: congenital; present acquired) (Figure 2).
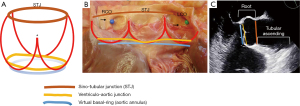
Definition of the aortic root complex
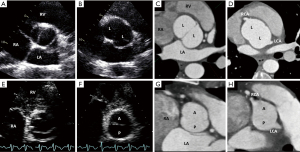
The term aortic root refers only to the most proximal part of the ascending thoracic aorta, from the distal end of the left ventricular outflow tract to the sinotubular junction (STJ), formed by the sinuses of Valsalva and containing the aortic valve (34) (Figure 3). The anatomy and physiology of the aortic root complex and its interaction with the valve have been thoroughly investigated as contemporary techniques for aortic valve repair have been introduced and more widely adopted (33,35). Functionally, and particularly, in relation to the competency of the BAV and surgical repair, three elements of the aortic root complex cooperate in determining physiological valve dynamics (36): (I) the STJ; (II) the aortic sinuses with the crown-like attachment line of the aortic valve cusps to the aortic wall at the aortic sinuses (which, as mentioned, assumes a peculiar form in the fused BAV, with 1 of the 3 “crown tips” corresponding to the under-the-raphe pseudocommissure, reaching a lower height than the other 2) (Figure 3); and (III) the aortic annulus, which is a virtual circular line inside the left ventricular outflow tract, running through the nadir of the aortic cusps and the bases of the respective inter-cusp triangles (Figure 3). The aortic annulus is a virtual surrogate for the ventriculo-aortic junction, which is the real boundary of the aortic root complex, identified anatomically as the transition from the ventricular muscle to the aortic media and located circumferentially slightly above the nadir of the aortic cusps, crossing the semilunar lines of each cusp’s attachment (Figure 3). However, for both surgical and imaging purposes, the virtual aortic annulus is the practical and clinically used anatomical landmark. The aortic root complex, particularly the size of the aortic annulus and the STJ, are indispensable in the maintenance of sufficient diastolic cusp coaptation area to prevent the progression of aortic regurgitation (37) and its recurrence after surgery (38). Therefore, the aortic root complex is the anatomical scaffold that maintains BAV competency, with the BAV cusps acting as a stentless valve and the root complex as its native stent (36).
BAV types and specific phenotypes by the international consensus
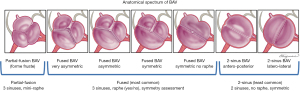
There are three BAV types: the fused BAV, the two-sinus BAV and the partial-fusion BAV, each with specific phenotypes (1-5).
The fused BAV type
The fused BAV type is the most common (Figure 4), accounting for approximately 90−95% of BAV cases (6,32). The fused BAV is characterized by two of the three cusps anatomically fused or jointed within three distinguishable aortic sinuses, resulting in two functional cusps (one fused or conjoined and the other non-fused) that are usually different in size and shape. Commonly, patients with a fused BAV demonstrate eccentric dominance of the non-fused aortic sinus and its cusp (compared to the other two sinuses and two fused cusps), irrespective of age (39). There are three specific BAV phenotypes within the fused type: right-left cusp fusion, right-non (non-coronary) cusp fusion and left-non cusp fusion (Figure 4). The right-left cusp fusion phenotype is the most common (70–80%) (6,32,40). The right-left cusp fusion phenotype is also the most common across all variations of aortic phenotypes (normal aorta, dilated ascending aorta, dilated root, dilated arch) and across valve dysfunction (regurgitation or stenosis). Although this right-left fusion phenotype statistically develops more AS (6), it has been associated in some patients (41,42) with aortic root dilatation, aortic regurgitation and male preponderance (these associations have been termed the “root phenotype”) (38,39). The right-left cusp fusion is also strongly associated with aortic coarctation in children (43).
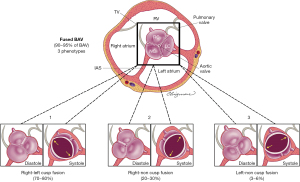
The right-non cusp fusion phenotype is the next most common (20–30%). It is associated with a higher prevalence of AS in adults (44) and also, independently predicts aortic regurgitation progression in adults (37). Similarly, the right-non cusp fusion phenotype is associated with a more rapid progression of AS and regurgitation in children and adolescents (43,45). The left-non cusp fusion phenotype is the least common phenotype (3–6%) across studies and needs further study. Referring to the fused phenotypes as BAV with right-left cusp fusion, right-non cusp fusion or left-non cusp fusion, is appropriate. Occasionally, it is possible to recognize a fused BAV but not to be able to discern the fusion phenotype, in which case BAV with indeterminate cusp fusion is appropriate.
Importance of raphe and valve symmetry in fused BAV phenotypes
Frequently (approximately 70%) but not always, a congenital fibrous ridge occurs between the fused cusps, termed “raphe” (32,46). The presence of a raphe has been associated with the progression of valvular dysfunction, particularly AS, and future valvular surgery (30,44,46). A raphe may be present but not initially visible on the echocardiogram and may become visible years later (47). The presence of a significantly calcified raphe may also make the repair of the regurgitant BAV more difficult or not possible. It is important to recognize that some fused BAVs may not have a congenital raphe (32) (this is not recognized by the Sievers’ classification). Therefore, when diagnosing a fused type, the next descriptor must be: with or without raphe, and further description of the raphe (i.e., calcified versus non-calcified) is warranted.
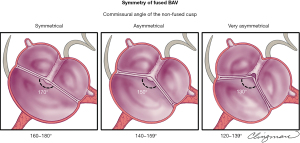
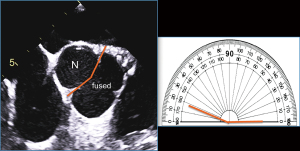
BAV symmetry for the fused BAV type is defined by the angle between the commissures for the non-fused cusp and, has recently become a critical aspect in the planning and performance of BAV repair for pure aortic regurgitation (5,11,48) (Figure 5). From a regurgitation-repair perspective, the BAV “concept” offers a single-line coaptation surface (a tricuspid aortic valve has 3 coaptation lines); if that single coaptation line is straight or almost straight (Figure 5, symmetrical), the repair of the regurgitant BAV is simple and reproducible. As the angle between the commissures of the non-fused cusp decreases <160° (48), the BAV becomes less symmetrical, resembling more a tricuspid (especially <140°) valve (Figure 5, very asymmetrical), which becomes technically more challenging for the surgeon to “bicuspidize” during the repair, yet remains repairable in experienced hands. Very asymmetrical valves may exhibit retraction of the free edge of the fused cusp at the raphe level, which is best appreciated by direct surgical visualization (Figure 5) or gross pathological inspection and not reliably by imaging. This retraction may contribute to valve regurgitation. Measuring the commissural angle of the non-fused cusp with TEE before cardiopulmonary bypass aids the surgeon in planning the repair (Figure 6). Therefore, the symmetry of a fused-type BAV is defined by the angle between the commissures of the non-fused cusp.
The two-sinus BAV type
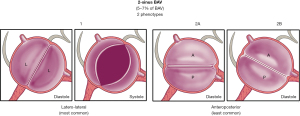
The two-sinus BAV type is uncommon, accounting for approximately 5–7% of BAV cases (5,6,32). In contrast to the fused type, the appearance of the two-sinus BAV does not suggest that two of the three cusps have fused; instead, it suggests that two cusps of roughly equal size and shape, each cusp occupying 180° of the annular circumference, were “formed” within only two aortic sinuses, resulting in a two-sinus/two-cusp valve (Figure 7) with 180° commissural angles. It is difficult to determine which two cusps could have coalesced to form a two-sinus BAV, but it is usually evident whether the cusps are laterolateral (side-to-side) or anteroposterior (front-to-back) within the short-axis base-of-the-heart plane (Figure 7); thus, these are the two specific phenotypes of the two-sinus BAV type. The two-sinus laterolateral BAV has one coronary artery arising from each sinus, whereas the anteroposterior BAV may have one coronary artery arising from each sinus or both coronary arteries arising from the anterior sinus (Figure 7). The two-sinus BAV likely represents a more severe expression of the embryologic mechanisms leading to the fused BAV. Referring to these phenotypes as two-sinus laterolateral BAV or two-sinus anteroposterior BAV is appropriate. Occasionally, despite suspicion, it may be difficult to be certain whether there are only two sinuses, in which case, terms such as possible or probable two-sinus BAV may be used. There is a lack of scientific data on the clinical/prognostic associations of the two-sinus BAV, which represents a “morphologically severe” form of BAV. As with all BAV phenotypes, the short axis tomographic cut is reproducible by MR and CT (Figure 8). Therefore, we hope that through this nomenclature/classification system, the research community directs more attention towards this BAV type.
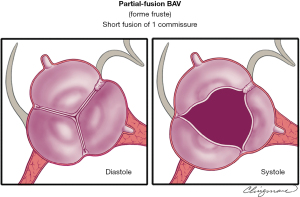
The partial-fusion (or forme fruste) BAV type
The prevalence of this recently recognized partial-fusion BAV (or forme fruste BAV) is unknown (49) (Figure 9). The appearance of the partial-fusion BAV is that of a typical tricuspid aortic valve with three symmetrical cusps with a systolic triangular opening and commissural angles of 120°, yet on surgical inspection or high-resolution imaging, less than 50% cusp fusion is noted at the base of a commissure, forming a small “mini-raphe” (5,49-51). It is important to recognize and further study the partial-fusion BAV, which has been described most frequently in the operating room in patients undergoing surgery for aortic dilatation (49). This forme fruste BAV results in alteration of aortic flow patterns, consisting of increased flow eccentricity and increased vortexes (50), perhaps partially explaining the apparent high prevalence of aortic dilatation in these patients. Referring to this phenotype as partial-fusion BAV or forme fruste BAV is appropriate, as is noting between which cusps the fusion occurs: right-left, right-non and so forth. This phenotype also requires further study.
Definition of aortic dilatation by the international consensus
The definition of “aortic aneurysm” (52) is rarely applied in clinical practice, and the term aneurysm carries a somber or dismal connotation for patients. Therefore, we propose a simple, universal term: aortic dilatation. Qualitative descriptive terms, such as saccular or fusiform dilatation or STJ effacement, may be important for aorta specialists and surgeons. A full discussion on aortic dilatation in patients with BAV is presented in the full documents (1-4).
BAV aortopathy phenotypes
The importance of recognizing BAV aortopathic phenotypes is that their presence and association with specific valvular phenotypes and dysfunction patterns may imply different clinical histories for a patient with BAV (53). There are three major forms of aortic dilatation phenotypes: (I) the ascending phenotype (dilatation preferentially located at the tubular ascending tract beyond the STJ (Figure 10), accounting for approximately 70% of BAV aortopathy cases; (II) the root phenotype [dilatation preferentially located at the root (sinuses of Valsalva)] accounting for approximately 20% of BAV aortopathy cases (Figure 10) (5,41,42,54). Importantly, the root phenotype may have mild ascending dilation but significantly prevails at the root, and the ascending phenotype may have mild root dilatation but significantly prevails at the ascending portion; (III) the extended phenotype, where significant dilatation may be present in both the root and tubular ascending aorta without predominance, or significant dilatation of the ascending and arch portions is found (Figure 10). The root and ascending phenotypes often correspond to two clearly distinct overall patient phenotypes: roughly, the older patient with BAV, either male or female, presenting more often with aortic valve sclerosis/stenosis and the ascending phenotype and, the younger patient with BAV, usually male, presenting with aortic regurgitation of degrees ranging from mild to severe and the root phenotype (41,55,56). However, those associations are not universal, and the right-left cusp fusion BAV can be associated with either aortic phenotype (56). The root phenotype has been associated with greater rates of acute aortic dissection in the postoperative follow-up of patients with BAV who had undergone simple aortic valve replacement compared to the ascending phenotype (57).
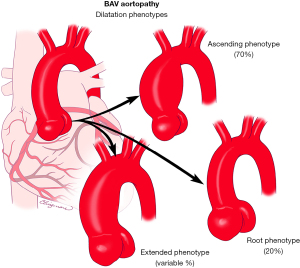
As mentioned, in a proportion of these cases, a localized dilatation at first observation can evolve during follow-up, with possible dilatation of previously normal adjacent segments of the aorta. In this scenario, the ascending phenotype can present, especially if a right-non cusp fusion valve is present, with associated dilatation of the aortic arch; it is appropriate to refer to this condition as ascending phenotype extended. Similarly, the root phenotype has been demonstrated to be independently associated with faster growth of the ascending tubular tract, so that cases of “cross-over” from an initial root phenotype configuration to significant dilatation of both tracts have been observed; root phenotype extended would be the appropriate definition of this form. In the context of a root phenotype, the presence and progression of effacement of the STJ may be an initial sign of this kind of evolution.
The BAV anatomic spectrum
The BAV phenotypic expression represents an anatomical continuum that is likely related to the severity of its embryologic mechanisms. Therefore, we propose a general BAV anatomical spectrum (Figure 11) of BAV phenotypes according to “bicuspidity”, defined as resemblance to a two-sinus BAV. This spectrum represents a continuum that begins with what is closer to a tricuspid aortic valve (i.e., partial-fusion), continues with the asymmetric fused phenotypes with raphe, and as the symmetry of the valve increases and raphes become absent, it finishes with the two-sinus phenotype.
Conclusions
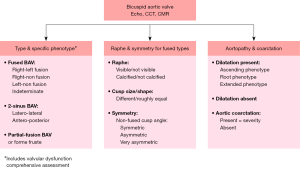
This international consensus identifies three major types of clinical presentation/outcomes for BAV patients, three major types of valvular phenotypes and three major types of aortopathy phenotypes. Based on the new nomenclature and classification consensus, Figure 12 presents a simple algorithm of the critical imaging evaluation for the BAV valvulo-aortopathy. Three critical anatomic aspects to be described in all patients with BAVs are: (I) the type and specific phenotype of the BAV and valve function; (II) the presence and characteristics of the raphe of the fused BAV, and the symmetry of the fused BAV; and (III) the presence and phenotype of aortopathy and whether coarctation is present.
Acknowledgments
The author wishes to thank Carl Clingman, MA, Senior Medical Illustrator, Biomedical & Scientific Visualization, Mayo Clinic, for his illustrations.
Funding: None.
Footnote
Conflicts of Interest: Artivion (Consulting fees, speaker), and Mayo co-PI of PROACT Xa trial, Biostable Science and Engineering, Inc. (Consulting fees, speaker). The disclosures are unrelated to the keynote lecture.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michelena HI, Corte AD, Evangelista A, et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Radiol Cardiothorac Imaging 2021;3:e200496. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. J Thorac Cardiovasc Surg 2021;162:e383-414. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Ann Thorac Surg 2021;112:e203-35. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg 2021;60:448-76. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. Speaking a common language: Introduction to a standard terminology for the bicuspid aortic valve and its aortopathy. Prog Cardiovasc Dis 2020;63:419-24. [Crossref] [PubMed]
- Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014;129:2691-704. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008;94:1634-8. [Crossref] [PubMed]
- Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150-61. [Crossref] [PubMed]
- Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg 2017;153:S65-71. [Crossref] [PubMed]
- de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Fernández B, Soto-Navarrete MT, López-García A, et al. Bicuspid Aortic Valve in 2 Model Species and Review of the Literature. Vet Pathol 2020;57:321-31. [Crossref] [PubMed]
- Slostad BD, Witt CM, O'Leary PW, et al. Unicuspid Aortic Valve: Demographics, Comorbidities, Echocardiographic Features, and Long-Term Outcomes. Circulation 2019;140:1853-5. [Crossref] [PubMed]
- Tsang MY, Abudiab MM, Ammash NM, et al. Quadricuspid Aortic Valve: Characteristics, Associated Structural Cardiovascular Abnormalities, and Clinical Outcomes. Circulation 2016;133:312-9. [Crossref] [PubMed]
- Fealey ME, Edwards WD, Miller DV, et al. Unicommissural aortic valves: gross, histological, and immunohistochemical analysis of 52 cases (1978-2008). Cardiovasc Pathol 2012;21:324-33. [Crossref] [PubMed]
- Naito S, Sequeira-Gross T, Petersen J, et al. Focus on a rare clinical entity: unicuspid aortic valve disease. Expert Rev Cardiovasc Ther 2020;18:625-33. [Crossref] [PubMed]
- Michelena HI, Suri RM, Katan O, et al. Sex Differences and Survival in Adults With Bicuspid Aortic Valves: Verification in 3 Contemporary Echocardiographic Cohorts. J Am Heart Assoc 2016;5:e004211. [Crossref] [PubMed]
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970;26:72-83. [Crossref] [PubMed]
- Michelena HI, Vallabhajosyula S, Prakash SK. Nosology Spectrum of the Bicuspid Aortic Valve Condition: Complex-Presentation Valvulo-Aortopathy. Circulation 2020;142:294-9. [Crossref] [PubMed]
- Niaz T, Poterucha JT, Johnson JN, et al. Incidence, morphology, and progression of bicuspid aortic valve in pediatric and young adult subjects with coexisting congenital heart defects. Congenit Heart Dis 2017;12:261-9. [Crossref] [PubMed]
- Niaz T, Poterucha JT, Olson TM, et al. Characteristic Morphologies of the Bicuspid Aortic Valve in Patients with Genetic Syndromes. J Am Soc Echocardiogr 2018;31:194-200. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Yang LT, Tribouilloy C, Masri A, et al. Clinical presentation and outcomes of adults with bicuspid aortic valves: 2020 update. Prog Cardiovasc Dis 2020;63:434-41. [Crossref] [PubMed]
- Padang R, Enriquez-Sarano M, Pislaru SV, et al. Coexistent bicuspid aortic valve and mitral valve prolapse: epidemiology, phenotypic spectrum, and clinical implications. Eur Heart J Cardiovasc Imaging 2019;20:677-86. [Crossref] [PubMed]
- Michelena HI, Katan O, Suri RM, et al. Incidence of Infective Endocarditis in Patients With Bicuspid Aortic Valves in the Community. Mayo Clin Proc 2016;91:122-3. [Crossref] [PubMed]
- Michelena HI, Chandrasekaran K, Topilsky Y, et al. The Bicuspid Aortic Valve Condition: The Critical Role of Echocardiography and the Case for a Standard Nomenclature Consensus. Prog Cardiovasc Dis 2018;61:404-15. [Crossref] [PubMed]
- Tanaka R, Yoshioka K, Niinuma H, et al. Diagnostic value of cardiac CT in the evaluation of bicuspid aortic stenosis: comparison with echocardiography and operative findings. AJR Am J Roentgenol 2010;195:895-9. [Crossref] [PubMed]
- Gleeson TG, Mwangi I, Horgan SJ, et al. Steady-state free-precession (SSFP) cine MRI in distinguishing normal and bicuspid aortic valves. J Magn Reson Imaging 2008;28:873-8. [Crossref] [PubMed]
- Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Executive summary. J Thorac Cardiovasc Surg 2018;156:473-80. [Crossref] [PubMed]
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776-84. [Crossref] [PubMed]
- Angelini A, Ho SY, Anderson RH, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. J Thorac Cardiovasc Surg 1989;98:362-7. [Crossref] [PubMed]
- Sabet HY, Edwards WD, Tazelaar HD, et al. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999;74:14-26. [Crossref] [PubMed]
- de Kerchove L, Jashari R, Boodhwani M, et al. Surgical anatomy of the aortic root: implication for valve-sparing reimplantation and aortic valve annuloplasty. J Thorac Cardiovasc Surg 2015;149:425-33. [Crossref] [PubMed]
- Sievers HH, Hemmer W, Beyersdorf F, et al. The everyday used nomenclature of the aortic root components: the tower of Babel? Eur J Cardiothorac Surg 2012;41:478-82. [Crossref] [PubMed]
- Khelil N, Sleilaty G, Palladino M, et al. Surgical anatomy of the aortic annulus: landmarks for external annuloplasty in aortic valve repair. Ann Thorac Surg 2015;99:1220-6. [Crossref] [PubMed]
- El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21. [Crossref] [PubMed]
- Yang LT, Pellikka PA, Enriquez-Sarano M, et al. Stage B Aortic Regurgitation in Bicuspid Aortic Valve: New Observations on Progression Rate and Predictors. JACC Cardiovasc Imaging 2020;13:1442-5. [Crossref] [PubMed]
- Schneider U, Hofmann C, Aicher D, et al. Suture Annuloplasty Significantly Improves the Durability of Bicuspid Aortic Valve Repair. Ann Thorac Surg 2017;103:504-10. [Crossref] [PubMed]
- Stefek HA, Lin KH, Rigsby CK, et al. Eccentric Enlargement of the Aortic Sinuses in Pediatric and Adult Patients with Bicuspid Aortic Valves: A Cardiac MRI Study. Pediatr Cardiol 2020;41:350-60. [Crossref] [PubMed]
- Kong WKF, Regeer MV, Poh KK, et al. Inter-ethnic differences in valve morphology, valvular dysfunction, and aortopathy between Asian and European patients with bicuspid aortic valve. Eur Heart J 2018;39:1308-13. [Crossref] [PubMed]
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007;31:397-404; discussion 404-5. [Crossref] [PubMed]
- Detaint D, Michelena HI, Nkomo VT, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014;100:126-34. [Crossref] [PubMed]
- Fernandes SM, Sanders SP, Khairy P, et al. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol 2004;44:1648-51. [Crossref] [PubMed]
- Evangelista A, Gallego P, Calvo-Iglesias F, et al. Anatomical and clinical predictors of valve dysfunction and aortic dilation in bicuspid aortic valve disease. Heart 2018;104:566-73. [Crossref] [PubMed]
- Fernandes SM, Khairy P, Sanders SP, et al. Bicuspid aortic valve morphology and interventions in the young. J Am Coll Cardiol 2007;49:2211-4. [Crossref] [PubMed]
- Kong WK, Delgado V, Poh KK, et al. Prognostic Implications of Raphe in Bicuspid Aortic Valve Anatomy. JAMA Cardiol 2017;2:285-92. [Crossref] [PubMed]
- Yang LT, Enriquez-Sarano M, Michelena HI. The bicuspid aortic valve raphe: an evolving structure. Eur Heart J Cardiovasc Imaging 2020;21:590. [Crossref] [PubMed]
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85. [Crossref] [PubMed]
- Sperling JS, Lubat E. Forme fruste or 'Incomplete' bicuspid aortic valves with very small raphes: The prevalence of bicuspid valve and its significance may be underestimated. Int J Cardiol 2015;184:1-5. [Crossref] [PubMed]
- Guala A, Rodriguez-Palomares J, Galian-Gay L, et al. Partial Aortic Valve Leaflet Fusion Is Related to Deleterious Alteration of Proximal Aorta Hemodynamics. Circulation 2019;139:2707-9. [Crossref] [PubMed]
- Michelena HI, Yang LT, Enriquez-Sarano M, et al. The elusive 'forme fruste' bicuspid aortic valve: 3D transoesophageal echocardiography to the rescue. Eur Heart J Cardiovasc Imaging 2020;21:1169. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Della Corte A, Michelena HI, Citarella A, et al. Risk Stratification in Bicuspid Aortic Valve Aortopathy: Emerging Evidence and Future Perspectives. Curr Probl Cardiol 2021;46:100428. [Crossref] [PubMed]
- Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging 2013;6:1301-10. [Crossref] [PubMed]
- Della Corte A, Bancone C, Dialetto G, et al. The ascending aorta with bicuspid aortic valve: a phenotypic classification with potential prognostic significance. Eur J Cardiothorac Surg 2014;46:240-7; discussion 247. [Crossref] [PubMed]
- Wojnarski CM, Roselli EE, Idrees JJ, et al. Machine-learning phenotypic classification of bicuspid aortopathy. J Thorac Cardiovasc Surg 2018;155:461-469.e4. [Crossref] [PubMed]
- Girdauskas E, Disha K, Rouman M, et al. Aortic events after isolated aortic valve replacement for bicuspid aortic valve root phenotype: echocardiographic follow-up study. Eur J Cardiothorac Surg 2015;48:e71-6. [Crossref] [PubMed]






