Strategies in the surgical treatment of type A aortic arch dissection
Introduction
Aortic dissection involving the ascending aorta is a lethal condition, with mortality approaching 60% if surgical intervention is not performed early (1).
Stanford type A acute aortic dissection involves the ascending aorta, aortic arch and a variable extent of the descending thoracic aorta. Identification and resection of the primary intimal tear with re-approximation of the intima and adventitia remains the surgical principle of repair. Despite using this approach, a residual dissection flap persists in the arch and descending thoracic aorta in 64-90% of patients (2-5). This can lead to distal malperfusion in the acute setting (6), with a risk of aneurysm formation and rupture over the long term (7).
Despite the advancement of cardiac surgery techniques, employment of modern cerebral protection strategies, myocardial protection, and aortic graft development, the surgical mortality of these operations, even in centers with a specific interest in aortic surgery, is in the range of 25% (8). In the modern era of cardiac surgery, it is rare to undertake a “common” operation with a mortality of 25% and this serves as a challenge for the cardiac surgery community to improve. In order to reduce this high operative mortality, one first needs to understand the contributing factors.
Drivers of early mortality after surgical repair of type A dissection
Operative mortality for acute type A dissection is multifactorial, yet poorly elucidated. Review of our own database showed that the major classes of operative mortality are related to hemorrhage, cerebral ischemia, cardiogenic shock, and distal malperfusion. Perrault (2012, unpublished data) found cardiogenic shock, cerebral ischemia and massive hemorrhage to be responsible for almost 85% of perioperative deaths.
Visceral and renal malperfusion are often under-recognized at the time of presentation, with the emphasis placed on reconstructing the threatened proximal thoracic aorta and the associated valvular insufficiency. Distal malperfusion may manifest as ileofemoral, mesenteric, renal, cerebral or spinal cord in nature. The resulting lower limb ischemia, gut ischemia, renal failure and stroke significantly increases mortality.
It has been reported that as many as 20-30% of patients presenting with type A dissection may have one or more malperfusion syndromes (6,9). According to the data of the Pacini group (6), the overall mortality in type A dissection was almost 3 times higher in the group with malperfusion (43.7%) than with no malperfusion (15%).
This association of malperfusion and increased mortality is well documented (9-12). A recent prospective study on the use of hybrid operating rooms (OR) for the management of acute dissection found that a hybrid OR enabled the identification of downstream malperfusion sites (13). In this study, 23% of patients with acute type A dissection required primary endovascular intervention and 35% required descending aortic repair in addition to ascending aortic replacement. Visceral malperfusion, however, is challenging to diagnose in the acute and immediate post-operative setting and is most likely under-appreciated. Under-recognition of early visceral and renal ischemia may lead to mortality traditionally attributed to “multisystem organ failure”.
Once we better understand the drivers of early mortality, therapies can be guided to the individual problems. Massive hemorrhage, cerebral ischemia and visceral/peripheral malperfusion may be areas that can be targeted by newer therapies.
Drivers of late mortality after surgical repair of type A dissection
Long-term survival and freedom from dissection related events are in the range of 50% at 10 years (14) after type A repair. The rate of re-intervention in this patient group is in the range of 18-22% (15-18). Recently, there have been several provocative studies identifying variables in the sub-acute phase that may portend a poor long-term outcome. These variables include:
- Size of the descending thoracic aorta at initial presentation: Sakaguchi (19) has found that significant medically treated pre-operative hypertension as well as a descending aortic diameter greater than 35 mm are risk factors for a patent false-lumen (a marker of aortic dilatation) postoperatively.
- False-lumen patency: patency of the false lumen has been implicated as a risk factor in the fate of the residual aorta (20,21). This may result from incomplete resection of the primary intimal tear or more likely secondary re-entry sites downstream in the arch and aorta. Some evidence suggests that a partially thrombosed false lumen may in fact be a worse prognostic factor than a completely patent false lumen (22).
- False lumen diameter: Song et al. (7) found that an initial false-lumen maximal diameter ≥22 mm in the proximal descending aorta was associated with accelerated aortic dilation and catastrophic clinical events.
- Primary and residual intimal tear location and size: in a recent, thoughtful study, Evangelista et al. identified a large proximal intimal tear (>10 mm) as a significant risk factor for dissection related events and mortality (14).
Traditional surgical intervention
The current best practice guidelines for type A dissection repair involve ascending aortic replacement and open distal anastomosis (hemiarch) under circulatory arrest with varying degrees of hypothermia and selection of cerebral protection techniques including antegrade or retrograde cerebral perfusion, or deep hypothermia alone. Although short periods of circulatory arrest are very well tolerated (23), the prolonged periods of circulatory arrest that are occasionally required to reconstruct a dissected arch may account for the high incidence of neurologic complications following this operation. Cerebral ischemia may be present pre-operatively or can be a complication of the operation itself. As previously noted, cerebral ischemia is one of the drivers of this early mortality.
Another hypothetical cause of high mortality with traditional surgery is that the primary intimal tear is not always addressed. Patients who undergo sternotomy for repair are occasionally discovered to have a primary intimal tear in the arch with a flap that extends both retrograde and antegrade. In these cases, the option of emergency total arch replacement may be viewed as prohibitively risky and the primary intimal tear is not resected. Careful interpretation of the pre-operative CT, however, can identify the culprit primary intimal tear in 40-100% of patients (24,25).
More aggressive strategies to address the arch at the time of type A repair
For years, several reports from Japan have advocated a formal total arch and elephant trunk repair in acute dissection to mitigate the long-term complications of the residual dissection in the arch and more distal aorta (26,27).
Over the last decade, various strategies have been employed to decrease the risk of total arch replacement. Open total arch replacement and ascending aortic replacement with a “frozen” elephant trunk technique have been developed and advocated (28). Pochettino and colleagues (29) have advocated the placement of an antegrade stent-graft into the proximal descending aorta during open type A dissection repair.
This hybrid approach, of conventional arch replacement with downstream placement of a stent-graft through an open arch, has stimulated the development of novel grafts such as the E-vita prosthesis, which is composed of a Dacron segment for replacement of the ascending aorta and arch, together with a stent graft segment, which is positioned in the proximal descending thoracic aorta. The group at Essen (30) has developed the International E-vita open registry with promising early results.
Although these approaches have been shown to promote false lumen thrombosis and may decrease long-term complications, they require systemic hypothermia and prolonged periods of circulatory arrest to perform the conventional total arch replacement. While early results indicate the techniques to be “safe” when compared to conventional surgery for acute aortic dissection, operative morbidity and mortality remain substantial.
Novel and innovative hybrid strategies to address the arch at the time of type A repair
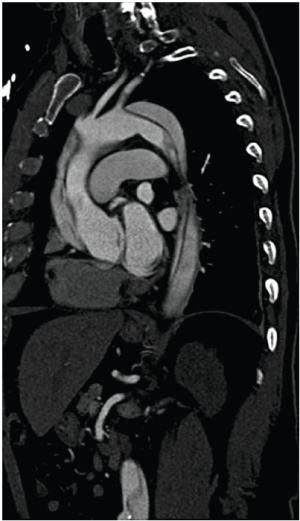
Several groups have recently published novel single-stage hybrid techniques, involving ascending aortic replacement, arch de-branching and antegrade endovascular stent deployment (31-34). Based on a skill-set derived from our experience with elective thoracic aortomegaly patients (35), we have recently been performing surgery for acute type A dissection without circulatory arrest and consequently using mild hypothermia (32) (Figures 1-4). Using a very similar strategy, Chang et al. operated on 21 consecutive type A dissection patients with one hospital mortality (34).
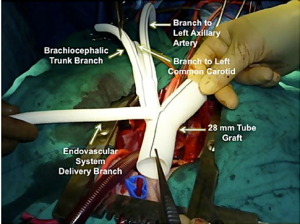
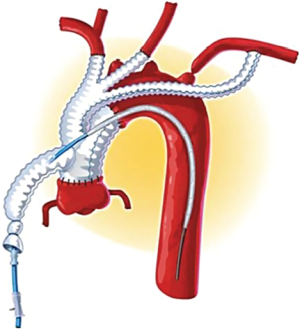

Marullo and colleagues (33) have employed an interesting staged approach whereby the ascending aorta is replaced and the arch is de-branched. An endograft is then deployed in the arch and proximal descending thoracic aorta if clinically indicated by the presence of a malperfusion syndrome or early evidence of distal aortic dilation. Using this strategy they deployed an endograft in 62.5% of patients, resulting in false lumen thrombosis and no resultant endoleaks.
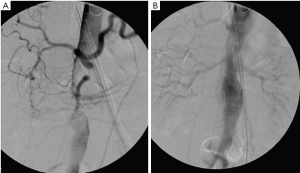
Figure 5 shows an intra-operative aortogram of a 54-year old male who presented with an arch dissection starting at the left carotid artery. The pre-operative CT revealed the common finding of a severely effaced true lumen in the distal descending and thoraco-abdominal aorta. The clinical significance of this finding of an “effaced true lumen” remains unclear. After undergoing ascending aortic replacement and arch de-branching, and before thoracic endovascular aneurysm repair (TEVAR) placement, an intra-operative angiogram was done showing severely compromised flow to visceral vessels and kidneys (Figure 5A). Placement of an endovascular device across the arch improved flow to the abdominal aorta and visceral organs (Figure 5B). Traditional hemiarch replacement would have left the patient with compromised flow to the viscera.
There are no randomized studies comparing “extended arch” strategies to conventional “open distal anastomosis” technique for acute type A dissection surgery. However, potential advantages of a hybrid repair technique include:
- Absence of deep hypothermia and associated coagulopathy/hemorrhage
- Arch reconstruction is made into an easier, more “proximal” operation, thus potentially decreasing hemorrhagic complications
- Complete resection/exclusion of primary intimal tear
- Improved recognition and treatment of acute malperfusion
- Decreased cerebral ischemia by avoiding prolonged circulatory arrest
- Elimination of residual tears in the arch which are a risk factor for aortic complications
- Provides a suitable proximal landing zone if future distal endovascular treatment is required
Future strategies to address the arch at time of type A repair
The development of endovascular techniques has allowed surgeons to pursue novel strategies in an effort to further reduce the risk associated with surgery for acute type A dissection, and to address the residual type B dissection.
As experience with deploying stents in the proximal aorta increases, isolated endovascular repair of type A dissection may become a realistic possibility. Two recent studies evaluated pre-operative CT findings and found that up to 30-50% of patients may be candidates for an isolated endovascular approach (24,25). Anecdotal case reports of isolated TEVAR for acute type A dissection have been published (36), leading to the possibility of isolated ascending aortic TEVAR becoming a viable treatment option for patients previously considered too high risk for surgery.
Availability of off-the-shelf branched endovascular grafts and a hybrid suite may further advance the management of acute aortic dissection. In the future, we envision a strategy of complete endovascular repair in a proportion of cases. Whether this can improve short- and long-term results will need to be determined. We propose that a second, more likely option might involve open ascending aortic replacement with Dacron graft, and formal surgical attention to valve reconstruction, coronary arteries and pericardial effusion followed by branched endovascular repair of the arch. Timing of endovascular deployment could be immediate at the time of ascending aortic replacement or delayed based on a more refined understanding of risk factors for early and late complications. This type of strategy would simplify the current hybrid operations by removing the need to further de-branch the arch. Strategies such as these emphasize the need for cardiac surgeons to be trained in endovascular aortic surgery and help define future treatment algorithms. At our center, all thoracic endovascular cases are done by a collaborative team consisting of interventional radiology, cardiac surgery, and cardiac anesthesiology. We have found benefits to having more than one perspective in the treatment of these patients.
Potential indications for arch replacement in acute type A dissection
The nuances of aortic dissection and risk factors for acute and long-term complications will continue to evolve. Based on the current understanding of aortic dissection, the following might be conditions where we would consider arch replacement at time of type A surgery:
- Pre-existing arch aneurysm
- Primary intimal tear identified on pre-operative CT in the distal arch or descending thoracic aorta
- Secondary intimal tear in the arch measuring >10 mm
- Clinical signs of visceral or peripheral extremity malperfusion
- Radiologic signs of potential visceral, renal and peripheral compromise such as a severely effaced true lumen in descending thoracic aorta
- False lumen diameter > than 22 mm (20)
- Descending thoracic aorta diameter >35 mm
It is also worth questioning whether young age should be an indication for a more aggressive approach to the arch. On one hand, these patients have a more aggressive disease process and are at risk for complications over the years, while on the other hand, the long-term outcome of an endovascular prosthesis in the angulated arch has not been delineated. Review of our local database revealed one late complication in 15 patients with Zone 0 TEVAR at up to 47 months of follow up, with mean radiological follow up of 545 days and cumulative follow up of 8,176 days (in submission).
Conclusions
Strides made in understanding causes of early and late mortality following conventional type A repair will serve to reduce the morbidity and mortality associated with this lethal condition. Continued substantial advancement in outcomes will be made when risk factors for operative mortality and long-term complications are further elucidated, understood and validated.
A detailed and precise analysis of pre-operative CT will become the cornerstone in the assessment of these risk factors. Specifically, the determination of the site and size of the primary and secondary intimal tears, as well as characterization of distal perfusion and the size of both true and false lumens, will be critical in pre-operative planning.
A more nuanced approach to the aortic arch may be justified in those patients that are at risk for complications, with the axiom that any adjunctive procedure would substantially reduce mortality. However, much yet needs to be discerned regarding the optimal techniques to address the arch, methods of cerebral protection and perfusion, and the types of de-branching and endovascular procedures chosen.
Theoretically, aortic arch replacement at the time of type A dissection repair has the advantage of reducing early (malperfusion and addressing arch tears) and late (aneurysm expansion) morbidity and mortality. There is no unequivocal evidence to support this assertion and further research is required to better understand which patients would benefit.
A hybrid arch repair approach has the potential to reduce operative morbidity and mortality as compared to a formal surgical total arch replacement technique by moving suture lines more proximally, and avoiding prolonged circulatory arrest and profound hypothermia. These hybrid techniques and future endovascular and branched endovascular techniques need to be studied for their potential impact on short- and long-term mortality rates.
We believe that the arch should very likely be addressed at the time of type A surgery, with the caveat that the aortic surgery community is able to identify which patients are at risk for both poor short- and long-term outcomes and if the adjunct procedure can be performed without increased risk and preferably a reduction in mortality. In early 2013, the answers to the above questions are not completely understood. Thus, the arch should only be replaced at time of type A dissection in certain centers with experience in aortic surgery, interest in verifying the known risk factors, curiosity to assess new prognostic indicators and an ability to academically report their findings.
Our continued mandate should be to further elucidate which patients will benefit from arch replacement and assess these results in the context of the various techniques available to us as they evolve, with the ultimate goal of decreasing short- and long-term mortality rates in those with type A dissection.
Acknowledgements
The authors would like to thank Drs. William Kent, Eric Herget and Kishan Narine for their help in reviewing and editing the manuscript.
Disclosure: Dr. Appoo discloses consultancy relationship with Gore Medical.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903.
- Kimura N, Tanaka M, Kawahito K, et al. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2008;136:1160-6, 1166.e1-3.
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50.
- Tan ME, Morshuis WJ, Dossche KM, et al. Long-term results after 27 years of surgical treatment of acute type a aortic dissection. Ann Thorac Surg 2005;80:523-9.
- Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479-86; discussion 486-7.
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112-22.
- Pacini D, Leone A, Belotti LM, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg;32:255-62.
- Deeb GM, Williams DM, Bolling SF, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg 1997;64:1669-75; discussion 1675-7.
- Girdauskas E, Kuntze T, Borger MA, et al. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg 2009;138:1363-9.
- Patel HJ, Williams DM, Dasika NL, et al. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg 2008;135:1288-95; discussion 1295-6.
- Tsagakis K, Konorza T, Dohle DS, et al. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg 2013;43:397-404.
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41.
- Fattori R, Bacchi-Reggiani L, Bertaccini P, et al. Evolution of aortic dissection after surgical repair. Am J Cardiol 2000;86:868-72.
- Kirsch M, Soustelle C, Houël R, et al. Risk factor analysis for proximal and distal reoperations after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2002;123:318-25.
- Concistrè G, Casali G, Santaniello E, et al. Reoperation after surgical correction of acute type A aortic dissection: risk factor analysis. Ann Thorac Surg 2012;93:450-5.
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64.
- Sakaguchi G, Komiya T, Tamura N, et al. Patency of distal false lumen in acute dissection: extent of resection and prognosis. Interact Cardiovasc Thorac Surg 2007;6:204-7.
- Immer FF, Krähenbühl E, Hagen U, et al. Large area of the false lumen favors secondary dilatation of the aorta after acute type A aortic dissection. Circulation 2005;112:I249-52.
- Park KH, Lim C, Choi JH, et al. Midterm change of descending aortic false lumen after repair of acute type I dissection. Ann Thorac Surg 2009;87:103-8.
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59.
- Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804.
- Patel HJ, Nguyen C, Diener AC, et al. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417-23.
- Moon MC, Greenberg RK, Morales JP, et al. Computed tomography-based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. J Vasc Surg 2011;53:942-9.
- Sobocinski J, O’Brien N, Maurel B, et al. Endovascular approaches to acute aortic type A dissection: a CT-based feasibility study. Eur J Vasc Endovasc Surg 2011;42:442-7.
- Ando M, Takamoto S, Okita Y, et al. Elephant trunk procedure for surgical treatment of aortic dissection. Ann Thorac Surg 1998;66:82-7.
- Konishi T, Higuchi K, Fukata M, et al. Extended aortic replacement in acute dissection by the separated elephant trunk technique. Ann Thorac Surg 1999;67:1664-8.
- Baraki H, Hagl C, Khaladj N, et al. The frozen elephant trunk technique for treatment of thoracic aortic aneurysms. Ann Thorac Surg 2007;83:S819-23; discussion S824-31.
- Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9; discussion 489-90.
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4.
- Diethrich EB, Ghazoul M, Wheatley GH, et al. Surgical correction of ascending type a thoracic aortic dissection: simultaneous endoluminal exclusion of the arch and distal aorta. J Endovasc Ther 2005;12:660-6.
- Kent WD, Herget EJ, Wong JK, et al. Ascending, total arch, and descending thoracic aortic repair for acute DeBakey type I aortic dissection without circulatory arrest. Ann Thorac Surg 2012;94:e59-61.
- Marullo AG, Bichi S, Pennetta RA, et al. Hybrid aortic arch debranching with staged endovascular completion in DeBakey type I aortic dissection. Ann Thorac Surg 2010;90:1847-53.
- Chang Q, Tian C, Wei Y, et al. Hybrid total arch repair without deep hypothermic circulatory arrest for acute type A aortic dissection (R1). J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Kent WD, Wong JK, Herget EJ, et al. An alternative approach to diffuse thoracic aortomegaly: on-pump hybrid total arch repair without circulatory arrest. Ann Thorac Surg 2012;93:326-8.
- Metcalfe MJ, Karthikesalingam A, Black SA, et al. The first endovascular repair of an acute type A dissection using an endograft designed for the ascending aorta. J Vasc Surg 2012;55:220-2.





